Asymmetric Aldol/Vinylogous Aldol Reaction Catalyzed by Chiral Phosphine Oxide: Stereoselective Synthesis of 4-Pyranones
Diunggah oleh
Nathan Ray AlimJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Asymmetric Aldol/Vinylogous Aldol Reaction Catalyzed by Chiral Phosphine Oxide: Stereoselective Synthesis of 4-Pyranones
Diunggah oleh
Nathan Ray AlimHak Cipta:
Format Tersedia
Asymmetric Aldol/Vinylogous Aldol Reaction Catalyzed by Chiral Phosphine Oxide:
Stereoselective Synthesis of 4-Pyranones
Nathan Ray Alim,1 Shunsuke Kotani, 1,2 Shiki Miyazaki, 1 Yasushi Shimoda,1 Masaharu Sugiura,1 Makoto Nakajima 1
1Graduate School of Pharmaceutical Sciences, Kumamoto University
2Priority Organization for Innovation and Excellence, Kumamoto University
E-mail: nakajima@gpo.kumamoto-u.ac.jp
1. Abstract 3. Background: 4-Pyranone Construction
Enantioselective Aldol/Vinylogous Aldol Reaction: 4-Pyranone Synthesis
Enantioselective Branched Double Aldol Reaction
(S)-BINAPO
(10 mol %) O (S)-BINAPO (10 mol %)
O
SiCl4 (3.0 eq) Ph SiCl4 (4.0 eq) O OH
OMe O O iPr NEt (5.0 eq)
OH P
2 Ph O O iPr NEt (5.0 eq)
+ 2 up to dr = 97/3
Ph + Ar ∗ R
Ar H CH 2Cl 2 (0.5 M) Ar O Ar Ar CH 2Cl 2/EtCN, –60 °C, 24 h ∗ up to 97% ee
P R H
–60 ˚C, 24 h Ph HO R
(4.0 eq) O
two stereocenters
up to 70% yield Shimoda, Y.; Kotani, S.; Sugiura, M.; Nakajima, M. Chem. Eur. J. 2011, 17, 7992.
3 new bonds and 2 stereocenters up to 98% ee (S)-BINAPO
are constructed in one step. 2,3-dihydro-4-pyranones
4-Previous Work: Branched type Double Aldol Reaction O
Ph
P
2. 4-Pyranones (S)-BINAPO (10 mol %)
SiCl4 (1.5 eq.) O OH
Ph
O H Ph
4-Pyranone: Structure, Biological Activities, Previous Synthesis Scheme + PhCHO Cy 2NMe (10 eq.) P
Ph Ph
MeO CH 2Cl 2 / EtCN (1 / 1) O
O
Biologically active 4-pyranone derivatives
4-Pyranone
(2.5 eq.)
–60 °C, 24 h O Ph (S)-BINAPO
O F 3C Cy = Cyclohexyl
NH 1st aldol
O Stereoselective
reaction
O O Enolization Cl3 cyclization
HN NH 2 S Cl3
O at α-position Si
Pyran ring Ketone 4-Pyranone O Si Cl3Si O OSiCl 3
HN HN Cy2NMe PhCHO
OH O O O O
O HO MeO Ph
Tipranavir MeO Ph 2nd aldol
O CO2H O MeO Ph
(anti-HIV) reaction
OH Cl3SiO Ph
MeO OMe Zanamivir
O ∗
(antiviral) O OH THIS WORK: Aldol/Vinylogous Aldol Reaction
HO Curcuminoid OH O
(S)-BINAPO (10 mol %)
(anticancer)
OMe O SiCl4 (3.0 eq.) OH
iPr NEt (5.0 eq.)
Previous synthetic strategy + PhCHO 2
CH 2Cl 2, –60 °C, 24 h Ph O Ph
Yamamoto (1988)
SiAr3 Cyclization
O 1st aldol Cl3
OSiMe 3 1) catalyst (10 mol %) Cl3
O O reaction Si Cl3
Me toluene, 0 °C, 2 h Me Me Cl3Si Si
+ iPr SiCl3 Si
MeO Al Me OMe O O 2NEt OMe O O PhCHO
Ph H 2) CF3CO2H O OMe O O
Me O Ph O Ph Ph
93% yield Ph Ph
J. Am. Chem. Soc. 1988, 110, 310. 97% ee SiAr3
Enolization Vinylogous-Aldol
catalyst
at γ-position Reaction
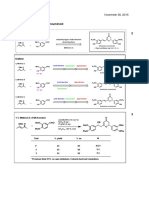
4. Aldol/Vinylogous Aldol Reaction
Optimization of Reaction Conditions Substrate Scope: Aldehyde
O
Ph
(S)-BINAPO (10 mol %) O P (S)-BINAPO (10 mol %) O
Ph
SiCl4 (3 eq.) SiCl4 (3.0 eq.)
OMe O iPr NEt (5 eq.)
OH Ph OMe O iPr NEt (5.0 eq.)
OH
+ 2 P + 2
PhCHO Ph RCHO
CH 2Cl 2 Ph O Ph O CH 2Cl 2 (0.05 M), –60 °C, 24 h R O R
(X eq.) (S)-BINAPO (4.0 eq.)
O O O
Entry X Amine Conc. [M] Temp. [°C] Time [h] Yield [%] Ee [%]
OH OH OH
1 2.5 iPr
2NEt 0.1 –40 3 36 82 O O O
2 2.5 iPr
2NEt 0.1 –60 10 55 85 Br Br
51% yield 70% yield 28% yield
98% ee 96% ee 85% ee
3 2.5 iPr
2NEt 0.1 –78 48 24 87
O O
4 2.5 iPr
2NEt 0.05 –60 13 43 93 OH
OH
5 4 iPr
2NEt 0.05 –60 24 51 98 O O
6 4 Cy 2NMe 0.05 –60 24 60 82 H 3CO OCH3
48% yield 35% yield
96% ee 93% ee
7 4 Cy 2NEt 0.05 –60 24 56 86
8 4 nBu
3N 0.05 –60 24 15 42 Substrate Scope: Enone
9 4 iBu 0.05 –60 24 0 - (S)-BINAPO (10 mol %) O
3N
SiCl4 (3.0 eq.)
OR O iPr NEt (5.0 eq.) OH
+ PhCHO 2
CH 2Cl 2 (0.05 M), –60 °C, 24 h Ph O Ph
Screening of catalysts (4.0 eq.)
chiral phosphine oxide (10 mol %)
SiCl4 (3.0 eq.) O
iPr NEt (5.0 eq.)
OMe O 2 Entry Leaving Group [OR] Yield [%] Ee [%]
+ PhCHO OH
CH 2Cl 2 (0.05 M), –60 °C, 24 h O
Ph
(4.0 eq.) Ph O Ph 1 MeO 51 98 P
Ph
2 47 93 Ph
CN tBu EtO P
Ph
O O
O O O 3 TMSO trace -
Ph Ph Ph (S)-BINAPO
P P P
Ph Ph O Ph 4 trace -
AcO
Ph Ph O Ph
P P P
Ph Ph Ph
O O
O O Possibe Reaction Mechanism
*
CN tBu
P P
51% yield 26% yield 26% yield O O Cl3
Br TMS (S)-BINAPO
98% ee 68% ee 89% ee Cl2Si SiCl4 Cl3Si Si
SiCl4
i
O O OMe O iPr NEt
2
OMe O O OMe O OSiCl3 Pr 2NEt MeO O O PhCHO
Ph Ph
P P
Ph Ph PhCHO Ph Ph Ph
Ph Ph Enolization
1st Aldol Reaction
P P at g-position
Ph Ph
O O
Cl3 Cl3 OSiCl3 O
Br TMS O Si Si OMe Work-up
Cl3SiO O OSiCl3
Ph MeO O O Cl3SiO OH
53% yield 43% yield
H Ph Ph
90% ee 89% ee Ph Ph O Ph Ph O Ph
MeO
2nd Aldol Reaction Cyclization SiCl3
(Vinylogous aldol)
Anda mungkin juga menyukai
- Introduction: Enantioselective Double Aldol Reaction 1. Abstract and Introduction 2. Background: Conventional Synthetic SchemesDokumen1 halamanIntroduction: Enantioselective Double Aldol Reaction 1. Abstract and Introduction 2. Background: Conventional Synthetic SchemesNathan Ray AlimBelum ada peringkat
- Japan Pharm SocDokumen11 halamanJapan Pharm SocNathan Ray AlimBelum ada peringkat
- Central Core Uprolides A Survey of Some Ring Closing Metathesis ApproachesDokumen6 halamanCentral Core Uprolides A Survey of Some Ring Closing Metathesis Approachessunaina agarwalBelum ada peringkat
- 2018 PDFDokumen32 halaman2018 PDFDicky Tak Hin WongBelum ada peringkat
- Anand SRF PPT 27-09-19Dokumen17 halamanAnand SRF PPT 27-09-19Munakala AnandaraoBelum ada peringkat
- Problem Session RemiDokumen1 halamanProblem Session RemiStudent BpharmaBelum ada peringkat
- Unsual Mecanismo DFT Arcilla LeerDokumen7 halamanUnsual Mecanismo DFT Arcilla LeerJosé Guadalupe García EstradaBelum ada peringkat
- Total Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachDokumen2 halamanTotal Synthesis of ( ) - Sinulariadiolide. A Transannular ApproachChem MistryBelum ada peringkat
- Total Synthesis of Chelidonine and Norchelidonine Via An Enamide - Benzyne - (2+2) Cycloaddi?on CascadeDokumen24 halamanTotal Synthesis of Chelidonine and Norchelidonine Via An Enamide - Benzyne - (2+2) Cycloaddi?on CascadeNgô Tuấn KiệtBelum ada peringkat
- Catalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Dokumen2 halamanCatalytic Asymmetric Total Synthesis of Exiguolide: Chem. Eur. J. 2020, Accepted Manuscript Doi:10.1002/chem.202001773Chem MistryBelum ada peringkat
- Selectivity in Organic SynthesiDokumen5 halamanSelectivity in Organic SynthesiChris LittleBelum ada peringkat
- Bioorthogonal Reactions For Labeling ProteinsDokumen1 halamanBioorthogonal Reactions For Labeling ProteinsTsung-Shing WangBelum ada peringkat
- Topic 16 Enols, Enamines, EnolatesDokumen12 halamanTopic 16 Enols, Enamines, EnolatesVijay SinghBelum ada peringkat
- Ethyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et ODokumen4 halamanEthyl 4 - (Triphenylphosphoranylidene) - Acetoacetate: O Tro Oh O Tro Co Et OPhạm Gia KhánhBelum ada peringkat
- Ch3e4 Stereoselective Synthesis MW Handout Reorganised 021111Dokumen51 halamanCh3e4 Stereoselective Synthesis MW Handout Reorganised 021111Kethavath VenkateshBelum ada peringkat
- Bahl & Bahl CH 36-40Dokumen99 halamanBahl & Bahl CH 36-40MAC MELLER JBelum ada peringkat
- A P V Platform For Oligonucleotide SynthesisDokumen12 halamanA P V Platform For Oligonucleotide Synthesismylove_withyou2001Belum ada peringkat
- Biskra Sayad RayeneDokumen1 halamanBiskra Sayad Rayenerayene sayadBelum ada peringkat
- Salsolene OxideDokumen1 halamanSalsolene OxideOrigamist KryaBelum ada peringkat
- Pentahetarenes With One Heteroatom: 1. General 2. SynthesesDokumen14 halamanPentahetarenes With One Heteroatom: 1. General 2. Synthesesangi gongopolBelum ada peringkat
- Photocatalysis in The Pharmaceutical Industry PDFDokumen26 halamanPhotocatalysis in The Pharmaceutical Industry PDFCamiloVerdugoBelum ada peringkat
- Novel Synthesis of Benzofuran - and Indol-2-Yl-Methanamine Derivatives PDFDokumen6 halamanNovel Synthesis of Benzofuran - and Indol-2-Yl-Methanamine Derivatives PDFMiguelAlejandroMantaChavezBelum ada peringkat
- Name Reactions 4Dokumen86 halamanName Reactions 4Ray CharlesBelum ada peringkat
- Archive of SID: Iranian Chemical SocietyDokumen5 halamanArchive of SID: Iranian Chemical SocietyashBelum ada peringkat
- A1 - Total Synthesis and Biological Evaluation of Marinopyrrole A and Analogs - n1Dokumen3 halamanA1 - Total Synthesis and Biological Evaluation of Marinopyrrole A and Analogs - n1tisun1002Belum ada peringkat
- Synthesis of Multisubstituted Furans, Pyrroles, and Thiophenes Via YnolatesDokumen4 halamanSynthesis of Multisubstituted Furans, Pyrroles, and Thiophenes Via YnolatesSaurav PaulBelum ada peringkat
- 金属有机化学课件 PDFDokumen408 halaman金属有机化学课件 PDFmingBelum ada peringkat
- 2017 Chem. Lett. 2017, 46, 1747-1750 Enantioselective RH - or Ir-Catalyzed Directed C (Sp3) H Borylation With Phosphoramidite Chiral LigandsDokumen4 halaman2017 Chem. Lett. 2017, 46, 1747-1750 Enantioselective RH - or Ir-Catalyzed Directed C (Sp3) H Borylation With Phosphoramidite Chiral LigandsHamza AnsarBelum ada peringkat
- Supporting Information: Sri Agustina, Masayoshi Tokuda, Hideto Minami, Cyrille Boyer, Per B. ZetterlundDokumen12 halamanSupporting Information: Sri Agustina, Masayoshi Tokuda, Hideto Minami, Cyrille Boyer, Per B. ZetterlundafifahBelum ada peringkat
- 2006 Paladium IsoxazolidinesDokumen4 halaman2006 Paladium Isoxazolidinesapi-19793040Belum ada peringkat
- Org. Synth. 2005, 81, 33-41 (Fer Discussion)Dokumen15 halamanOrg. Synth. 2005, 81, 33-41 (Fer Discussion)ludoBelum ada peringkat
- Group Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)Dokumen5 halamanGroup Meeting Problems 2021/12/11: Co Me N N Meo C Co Me Meo C CHCL 80 (R BN) Meoh, RT (2 Eq)dicky wongBelum ada peringkat
- TCI - Asymmetric OrganocatalystsDokumen8 halamanTCI - Asymmetric OrganocatalystsDeath Dealer61Belum ada peringkat
- Ebook Transition Metal Catalyzed Benzofuran Synthesis PDF Full Chapter PDFDokumen67 halamanEbook Transition Metal Catalyzed Benzofuran Synthesis PDF Full Chapter PDFdonald.winkle699100% (29)
- Low Temperature Asymmetric Epoxidation of Unfunctionalized Olefins Catalyzed by (Salen) MN (III) ComplexesDokumen4 halamanLow Temperature Asymmetric Epoxidation of Unfunctionalized Olefins Catalyzed by (Salen) MN (III) ComplexesAlejandro RodriguezBelum ada peringkat
- cm5b04726 Si 001Dokumen47 halamancm5b04726 Si 001VllsSBelum ada peringkat
- Work Summary: S O N S OODokumen4 halamanWork Summary: S O N S OOaazshaik5861Belum ada peringkat
- Enantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesDokumen10 halamanEnantioselective (3+3) Atroposelective Annulation Catalyzed by N-Heterocyclic CarbenesKatrin MarchenkoBelum ada peringkat
- Journal of Chemical Research Index Jan-Feb2021Dokumen12 halamanJournal of Chemical Research Index Jan-Feb2021mintillaBelum ada peringkat
- Scheme 1: Mechanism of The WeekDokumen12 halamanScheme 1: Mechanism of The WeekArianneBelum ada peringkat
- (Doi 10.1016/b978-0!12!386984-5.10001-1) Larkin, Peter - Infrared and Raman Spectroscopy - IntroductionDokumen5 halaman(Doi 10.1016/b978-0!12!386984-5.10001-1) Larkin, Peter - Infrared and Raman Spectroscopy - IntroductionCedric Omar Hdz RiescoBelum ada peringkat
- Transition Metal Catalyzed Benzofuran Synthesis 1St Edition Xiao Feng Wu Ebook Full ChapterDokumen51 halamanTransition Metal Catalyzed Benzofuran Synthesis 1St Edition Xiao Feng Wu Ebook Full Chaptertamera.stone860100% (6)
- Chem-353-Lecture 2Dokumen10 halamanChem-353-Lecture 2Caleb AsharleyBelum ada peringkat
- Synlett 2021, 32, 525-531Dokumen7 halamanSynlett 2021, 32, 525-531NoimurBelum ada peringkat
- Synthesis and Antibacterial Activity of 35methyl1phenyl1h123triazol4yl6aryl7h124triazolo34b134thiadiazineDokumen10 halamanSynthesis and Antibacterial Activity of 35methyl1phenyl1h123triazol4yl6aryl7h124triazolo34b134thiadiazineFinn NelsonBelum ada peringkat
- Jurnal DDSO Kelompok 3Dokumen6 halamanJurnal DDSO Kelompok 3vinny valleryBelum ada peringkat
- C C Bond Formation: Aldol Condensation - Aldol Condensation Initially GiveDokumen12 halamanC C Bond Formation: Aldol Condensation - Aldol Condensation Initially Giveaggelisgeorge8546Belum ada peringkat
- An Intramolecular, Ni (0) - Mediated Approach To The Nonracemic Biaryl Portion of VancomycinDokumen4 halamanAn Intramolecular, Ni (0) - Mediated Approach To The Nonracemic Biaryl Portion of VancomycinAndrés LópezBelum ada peringkat
- 2 - H-NMRDokumen38 halaman2 - H-NMRahmed mohamedBelum ada peringkat
- Molecules: Synthesis of Novel 3H-Quinazolin-4-ones Containing Pyrazolinone, Pyrazole and Pyrimidinone MoietiesDokumen11 halamanMolecules: Synthesis of Novel 3H-Quinazolin-4-ones Containing Pyrazolinone, Pyrazole and Pyrimidinone MoietiesAmer KasidehBelum ada peringkat
- European Journal of Medicinal Chemistry: Channamata Shankara Naveena, Poojary Boja, Nalilu Sucheta KumariDokumen12 halamanEuropean Journal of Medicinal Chemistry: Channamata Shankara Naveena, Poojary Boja, Nalilu Sucheta KumariWalid Ebid ElgammalBelum ada peringkat
- Synlett 2015, 26, 643-645Dokumen3 halamanSynlett 2015, 26, 643-645NoimurBelum ada peringkat
- 0040 4039 (84) 80121 5Dokumen4 halaman0040 4039 (84) 80121 5abcdefBelum ada peringkat
- 2000 Flash Vacuum Pyrolysis of 2-5-DiphenyloxazoleDokumen7 halaman2000 Flash Vacuum Pyrolysis of 2-5-Diphenyloxazolemuhammad noorBelum ada peringkat
- Fukuyama Group - Group Meeting Problems 07/04/2017Dokumen4 halamanFukuyama Group - Group Meeting Problems 07/04/2017Huỳnh ĐặngBelum ada peringkat
- ) Cychze: Synthesis of Benzo-Fused, 7,5-And 7,6-Fused Azepinones As Conformationally Restricted Dipeptide MimeticsDokumen4 halaman) Cychze: Synthesis of Benzo-Fused, 7,5-And 7,6-Fused Azepinones As Conformationally Restricted Dipeptide MimeticsAngie Melendez MendezBelum ada peringkat
- Proline-Catalyzed Intramolecular Cyclization of 5-Hydroxypentene To B-Halogenated TetrahydrofuranDokumen4 halamanProline-Catalyzed Intramolecular Cyclization of 5-Hydroxypentene To B-Halogenated TetrahydrofuranMarianaBelum ada peringkat
- Handbook of Coordination Catalysis in Organic ChemistryDari EverandHandbook of Coordination Catalysis in Organic ChemistryBelum ada peringkat
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973Dari EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973Belum ada peringkat
- Organometallic Chemistry: Plenary Lectures Presented at the Eighth International Conference on Organometallic Chemistry, Kyoto, Japan, 12-16 September 1977Dari EverandOrganometallic Chemistry: Plenary Lectures Presented at the Eighth International Conference on Organometallic Chemistry, Kyoto, Japan, 12-16 September 1977Y. IshiiBelum ada peringkat
- Midterm Chem4EngDokumen2 halamanMidterm Chem4EngNathan Ray Alim50% (2)
- Experiment 15. Acids, Bases, Salts and Buffers: Objective: 1. To Understand An Acid-Base ReactionDokumen6 halamanExperiment 15. Acids, Bases, Salts and Buffers: Objective: 1. To Understand An Acid-Base ReactionNathan Ray AlimBelum ada peringkat
- Experiment Seminar 11 PDFDokumen4 halamanExperiment Seminar 11 PDFNathan Ray AlimBelum ada peringkat
- Nathan Ray Alim, PH.DDokumen22 halamanNathan Ray Alim, PH.DNathan Ray AlimBelum ada peringkat
- A. Edta Titration: Group Standardization of Edta Mass Caco3 (G) Edta (ML) Aliquot (20/X) MLDokumen1 halamanA. Edta Titration: Group Standardization of Edta Mass Caco3 (G) Edta (ML) Aliquot (20/X) MLNathan Ray AlimBelum ada peringkat
- Prelim AnaChem LecDokumen1 halamanPrelim AnaChem LecNathan Ray AlimBelum ada peringkat
- Organic Chemistry Prelim Exam Part 1Dokumen1 halamanOrganic Chemistry Prelim Exam Part 1Nathan Ray AlimBelum ada peringkat
- Organic Chemistry 2 Chapter Quiz February 20, 2019 Write The Reaction Mechanism of The FollowingDokumen1 halamanOrganic Chemistry 2 Chapter Quiz February 20, 2019 Write The Reaction Mechanism of The FollowingNathan Ray AlimBelum ada peringkat
- ORGCHEM Lec 1-1Dokumen70 halamanORGCHEM Lec 1-1Nathan Ray AlimBelum ada peringkat
- OrgChem Lec 1-0Dokumen6 halamanOrgChem Lec 1-0Nathan Ray AlimBelum ada peringkat
- Victor 2Dokumen30 halamanVictor 2EmmanuelBelum ada peringkat
- Detail Design Drawings: OCTOBER., 2017 Date Span Carriage WayDokumen26 halamanDetail Design Drawings: OCTOBER., 2017 Date Span Carriage WayManvendra NigamBelum ada peringkat
- Angle Modulation: Hệ thống viễn thông (Communication Systems)Dokumen41 halamanAngle Modulation: Hệ thống viễn thông (Communication Systems)Thành VỹBelum ada peringkat
- Universitas Tidar: Fakultas Keguruan Dan Ilmu PendidikanDokumen7 halamanUniversitas Tidar: Fakultas Keguruan Dan Ilmu PendidikanTheresia Calcutaa WilBelum ada peringkat
- Unit 1 Module 3 Rep in PlantsDokumen26 halamanUnit 1 Module 3 Rep in Plantstamesh jodhanBelum ada peringkat
- Boundary Value Analysis 2Dokumen13 halamanBoundary Value Analysis 2Raheela NasimBelum ada peringkat
- СV Nestor RodriguezDokumen28 halamanСV Nestor RodriguezKate BrownBelum ada peringkat
- The Scope and Method of Economics: © 2007 Prentice Hall Business Publishing Principles of Economics 8e by Case and FairDokumen36 halamanThe Scope and Method of Economics: © 2007 Prentice Hall Business Publishing Principles of Economics 8e by Case and FairLangson phiriBelum ada peringkat
- De Thi Hoc Ki 1 Lop 11 Mon Tieng Anh Co File Nghe Nam 2020Dokumen11 halamanDe Thi Hoc Ki 1 Lop 11 Mon Tieng Anh Co File Nghe Nam 2020HiềnBelum ada peringkat
- The New Order of BarbariansDokumen39 halamanThe New Order of Barbariansbadguy100% (1)
- Jonathan Livingston Seagull - Richard Bach - (SAW000) PDFDokumen39 halamanJonathan Livingston Seagull - Richard Bach - (SAW000) PDFAdrià SonetBelum ada peringkat
- Highlights ASME Guides Preheat PWHT IDokumen4 halamanHighlights ASME Guides Preheat PWHT IArul Edwin Vijay VincentBelum ada peringkat
- DIR-819 A1 Manual v1.02WW PDFDokumen172 halamanDIR-819 A1 Manual v1.02WW PDFSerginho Jaafa ReggaeBelum ada peringkat
- COK - Training PlanDokumen22 halamanCOK - Training PlanralphBelum ada peringkat
- Yale Revision WorksheetDokumen3 halamanYale Revision WorksheetYASHI AGRAWALBelum ada peringkat
- PDFDokumen40 halamanPDFAndi NursinarBelum ada peringkat
- PM Jobs Comp Ir RandDokumen9 halamanPM Jobs Comp Ir Randandri putrantoBelum ada peringkat
- Word CountDokumen3 halamanWord CountLeo LonardelliBelum ada peringkat
- Worst of Autocall Certificate With Memory EffectDokumen1 halamanWorst of Autocall Certificate With Memory Effectapi-25889552Belum ada peringkat
- Sample REVISION QUESTION BANK. ACCA Paper F5 PERFORMANCE MANAGEMENTDokumen43 halamanSample REVISION QUESTION BANK. ACCA Paper F5 PERFORMANCE MANAGEMENTAbayneh Assefa75% (4)
- Coaxial Cable Attenuation ChartDokumen6 halamanCoaxial Cable Attenuation ChartNam PhamBelum ada peringkat
- DB Lecture Note All in ONEDokumen85 halamanDB Lecture Note All in ONEyonasante2121Belum ada peringkat
- Volvo BL 71 ManualDokumen280 halamanVolvo BL 71 ManualAlberto G.D.100% (2)
- Biotech NewsDokumen116 halamanBiotech NewsRahul KapoorBelum ada peringkat
- Alfa Week 1Dokumen13 halamanAlfa Week 1Cikgu kannaBelum ada peringkat
- Tangerine - Breakfast Set Menu Wef 16 Dec UpdatedDokumen3 halamanTangerine - Breakfast Set Menu Wef 16 Dec Updateddeveloper louBelum ada peringkat
- AIIMS 2015 Solved PaperDokumen436 halamanAIIMS 2015 Solved PaperSurya TejaBelum ada peringkat
- Module 6 Metal Properties and Destructive TestingDokumen46 halamanModule 6 Metal Properties and Destructive TestingMiki Jaksic100% (6)
- Lady in The House, Her Responsibilities & Ambitions: Amrita DuhanDokumen7 halamanLady in The House, Her Responsibilities & Ambitions: Amrita DuhanFitness FableBelum ada peringkat