ch14 Summary
Diunggah oleh
api-4654218090 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
2K tayangan1 halamanJudul Asli
ch14 summary
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
2K tayangan1 halamanch14 Summary
Diunggah oleh
api-465421809Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 1
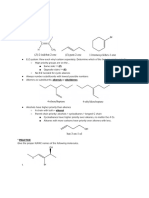
Summary of CH14 Delocalized Systems VI.
Allylic Grignard and allylithium reagents
I. Definition • Preparations
X
Delocalized systems: extended overlap of three or more contiguous p orbitals Mg/Et2O
R2R1C C CH2 R2R1C C CH2MgX
bond bond H H
allylic halide allylic Grignard
p orbital
R2R1C CH CH3 n-BuLi/TMEDA R2R1C C CH2Li
H
allyllithium
• Allylic Grignard and allyllithium reagents react with electrophiles
Allylic systems (C+, C•, C–) Conjugated dienes like other organometallic compounds.
II. Stability of delocalized -systems
• -electron delocalization stabilizes the entire -system VII. Conjugated Dienes
Synthesis The Heck Rection
C+: substituted allylic > 3° > allyl > 2° > 1°
Pd, or Ni
C•: substituted allylic or allyl > 3° > 2° > 1° CH2 CHCl + CH2 CH2
C–: CH2=CH-CH2– > CH3-CH2-CH2–
• Review resonance theory
VIII. Electrophilic 1,2 vs 1,4-additions of H-X or X2 to conjugated
-know how to draw resonance structures and determine their relative
dienes
stabilities.
H
-explain the formal charge (C+, C•, C–) distribution in a delocalized H2C C C CH2
-system H
H–X X H
H2C C C CH2 H2C C C CH2 X– (or
-know how resonance structures contribute to the stability of the actual R R or X–X 1,2-addition
X)
H H
molecule H
(X: Cl, Br) (or X)
• MO theory H2C C C CH2
-numbers of AOs is equal to numbers of MOs H
X H H
-node and its relationship to stability of MO (or
1,4-addition
X)
-explain the formal charge (C+, C•, C–) distribution
according to individual MO • Ionic mechanism via a delocalized C+ intermediate
-Fill up the bonding MOs with electrons lowers the • 1,2-addition is the kinetic product while 1,4-addition is the
energy of the system (stabilization) thermodynamic product
IX. Diels-Alder cycloadditions
III. Kinetic vs. Thermodynamic control • Diels-Alder reaction is a concerted reaction with a high
• Kinetic control: the ratio of products of a reaction is determined by the stereospecificity
relative rate.
• Conditions favor kinetic control: short reaction time, lower reaction
temp, irreversible reactions
• Thermodynamic control: the ratio of products of a reaction that reaches
equilibrium is determined by the relative stabilities of the products
• Conditions favor thermodynamic control: longer reaction time, higher
reaction temperature, reversible reactions
IV. Allylic halogenation
Br2 (low conc. high temp.) • Kinetically controlled addition favors endo products while
H2C CH CH2 H2C CH CH2
or NBS/hv or ROOR thermodynamically controlled addition favors exo products.
H allylic halide Br • Most Diels-Alder reactions are reversible.
• Radical substitution (favored by the formation of an allylic radical) vs.
X. Intra molecular Electrocyclic reactions
ionic addition
• NBS in the presence of ROOR or hv (radical mechanism) gives exclusive
allylic bromination or hu
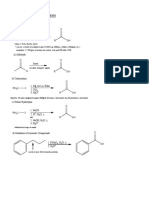
V. Nucleophilic substitutions
Nu
good Nu–
H2C C C
SN2 H R1 inversion
R1
R2 R2
H2C C C R1
H Nu
X poor Nu– R2
H2C C C H2C C C
SN1 H R1 H
R2 Nu
racemic mixture
1° or 2° allylic halides undergo SN1 reactions with poor nucleophiles
and SN2 reactions with good nucleophiles and 3° allylic halides
always undergo SN1 reactions.
Anda mungkin juga menyukai
- 118c Practice Synthesis KeyDokumen18 halaman118c Practice Synthesis Keyapi-465421809Belum ada peringkat
- Ca SynthDokumen1 halamanCa Synthapi-465421809Belum ada peringkat
- Phenol SynthDokumen1 halamanPhenol Synthapi-465421809Belum ada peringkat
- Benzene RxnsDokumen1 halamanBenzene Rxnsapi-465421809Belum ada peringkat
- 118c Practice Synthesis KeyDokumen18 halaman118c Practice Synthesis Keyapi-465421809Belum ada peringkat
- Ca RXN CheckDokumen1 halamanCa RXN Checkapi-4654218090% (1)
- Phenol Rxns IDokumen1 halamanPhenol Rxns Iapi-465421809Belum ada peringkat
- Amide RxnsDokumen1 halamanAmide Rxnsapi-465421809Belum ada peringkat
- Nitrile RxnsDokumen1 halamanNitrile Rxnsapi-465421809Belum ada peringkat
- Acyl Halide RxnsDokumen1 halamanAcyl Halide Rxnsapi-465421809Belum ada peringkat
- Amine SynthDokumen1 halamanAmine Synthapi-465421809Belum ada peringkat
- Anhydride RxnsDokumen1 halamanAnhydride Rxnsapi-465421809Belum ada peringkat
- Ester RxnsDokumen1 halamanEster Rxnsapi-465421809Belum ada peringkat
- Ca Rxns IIDokumen1 halamanCa Rxns IIapi-465421809Belum ada peringkat
- 118b mt1 Study Guide 2Dokumen21 halaman118b mt1 Study Guide 2api-465421809Belum ada peringkat
- Ca RXN CheckDokumen1 halamanCa RXN Checkapi-4654218090% (1)
- Ca SynthDokumen1 halamanCa Synthapi-465421809Belum ada peringkat
- Week 6 Practice Final Part 2 Chapter 18Dokumen4 halamanWeek 6 Practice Final Part 2 Chapter 18api-465421809Belum ada peringkat
- 118 BprsynthDokumen19 halaman118 Bprsynthapi-465421809Belum ada peringkat
- Ca Rxns IDokumen1 halamanCa Rxns Iapi-465421809Belum ada peringkat
- Week 5 Practice Final Part 1 - New Material 1Dokumen3 halamanWeek 5 Practice Final Part 1 - New Material 1api-465421809Belum ada peringkat
- 118b mt2 Study Guide 2Dokumen20 halaman118b mt2 Study Guide 2api-465421809Belum ada peringkat
- CH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - EduDokumen17 halamanCH 17: Aldehydes and Ketones: Nasiri CHE118B Final Study Guide Spring 2019 - S. Ly Sdly@ucdavis - Eduapi-465421809Belum ada peringkat
- Midterm 1 Practice QuestionsDokumen8 halamanMidterm 1 Practice Questionsapi-465421809Belum ada peringkat
- ch17 SummaryDokumen1 halamanch17 Summaryapi-465421809Belum ada peringkat
- ch15-16 SummaryDokumen1 halamanch15-16 Summaryapi-465421809Belum ada peringkat
- ch18 SummaryDokumen1 halamanch18 Summaryapi-465421809Belum ada peringkat
- MOC Alcohol RXN Map PDFDokumen2 halamanMOC Alcohol RXN Map PDFNickOoPandeyBelum ada peringkat
- Resonance Practice ProblemsDokumen12 halamanResonance Practice Problemsapi-465421809Belum ada peringkat
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Chem 132 2019 Tutorial QuestionsDokumen3 halamanChem 132 2019 Tutorial QuestionsYusuf Zaynab100% (1)
- SN1, SN2, E1 and E2 Reactions in OCDokumen60 halamanSN1, SN2, E1 and E2 Reactions in OCHimanshu SharmaBelum ada peringkat
- Thing To Remember - Haloalkane - StudentsDokumen10 halamanThing To Remember - Haloalkane - StudentspoornaBelum ada peringkat
- Ib PPT 10 HL PDFDokumen38 halamanIb PPT 10 HL PDFzarna nirmal rawalBelum ada peringkat
- C F C CL C - BR: HalogenoalkanesDokumen11 halamanC F C CL C - BR: HalogenoalkanesMufaro MutotiBelum ada peringkat
- Organic Chemistry Exercise PDFDokumen34 halamanOrganic Chemistry Exercise PDFBrightMoonBelum ada peringkat
- SN1 VS SN2 VS E1 VS E2Dokumen3 halamanSN1 VS SN2 VS E1 VS E2Lily LilonBelum ada peringkat
- A Level Chemistry Exemplars With CommentariesDokumen103 halamanA Level Chemistry Exemplars With CommentariesWeb Books0% (1)
- Alkyl HalidesDokumen26 halamanAlkyl Halidesharerambaghel906Belum ada peringkat
- 2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerDokumen12 halaman2018 PU2 H2 05 Halogen Derivatives Tutorial AnswerNeil SharmaBelum ada peringkat
- CHEM 203 Midterm Exam 2Dokumen7 halamanCHEM 203 Midterm Exam 2pBelum ada peringkat
- Flashcards - Topic 11 Kinetics - Edexcel IAL Chemistry A-LevelDokumen69 halamanFlashcards - Topic 11 Kinetics - Edexcel IAL Chemistry A-LevelzuveriaBelum ada peringkat
- S 2 Reaction (Substitution Nucleophilic Bimolecular)Dokumen15 halamanS 2 Reaction (Substitution Nucleophilic Bimolecular)Palash ChawhanBelum ada peringkat
- Isomerism: Definition-Structural Isomers: Same Molecular Formula Different Structures (Or Structural Formulae)Dokumen13 halamanIsomerism: Definition-Structural Isomers: Same Molecular Formula Different Structures (Or Structural Formulae)Trần Duy Tân100% (1)
- Industrial Chemistry - M.Sc.Dokumen34 halamanIndustrial Chemistry - M.Sc.Aniket ChowdhuryBelum ada peringkat
- Halides (Q.B) AdvancedDokumen26 halamanHalides (Q.B) AdvancedRaj ModiBelum ada peringkat
- Alkyl and Halide Ex. NeetDokumen39 halamanAlkyl and Halide Ex. NeetashishBelum ada peringkat
- Chemistry Haloalkanes and Haloarenes PDFDokumen40 halamanChemistry Haloalkanes and Haloarenes PDFGanesh KrishnaBelum ada peringkat
- sn1 sn2 E1 E2Dokumen2 halamansn1 sn2 E1 E2Anonymous ZAuWf2Belum ada peringkat
- $10, 11 Substitution of Alkyl HalidesDokumen14 halaman$10, 11 Substitution of Alkyl HalidesAnonymous 8ELpqXpHwxBelum ada peringkat
- Notes 02Dokumen67 halamanNotes 02Christine FernandezBelum ada peringkat
- 10hl.20.1 Types of Organic ReactionsDokumen74 halaman10hl.20.1 Types of Organic ReactionsKatarina VleugelsBelum ada peringkat
- SN1 Vs SN2 PDFDokumen1 halamanSN1 Vs SN2 PDFBhargavBelum ada peringkat
- Substitution Elimination FlowchartDokumen2 halamanSubstitution Elimination FlowchartAyah Al-AnaniBelum ada peringkat
- 7.alkil Halida (SN Dan E)Dokumen33 halaman7.alkil Halida (SN Dan E)akbar_rozaaqBelum ada peringkat
- Bachelor of Science in PCM: Singhania UniversityDokumen39 halamanBachelor of Science in PCM: Singhania UniversityChandresh mauryaBelum ada peringkat
- Organic Chemistry 11th Edition Ebook PDFDokumen41 halamanOrganic Chemistry 11th Edition Ebook PDFlouise.merrill249100% (36)
- Organic Chemistry sn2 sn1 E2 E1 PDFDokumen3 halamanOrganic Chemistry sn2 sn1 E2 E1 PDFMCHENLOLBelum ada peringkat
- Cuaderno Trabajo - 2019-1Dokumen39 halamanCuaderno Trabajo - 2019-1Isaac Farfan CondorBelum ada peringkat
- E1 and E2 EliminationDokumen5 halamanE1 and E2 EliminationHarveen HayerBelum ada peringkat