Plating 093
Diunggah oleh
Kimia0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
5 tayangan1 halamanTeknis
Hak Cipta
© © All Rights Reserved
Format Tersedia
DOCX, PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniTeknis
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
5 tayangan1 halamanPlating 093
Diunggah oleh
KimiaTeknis
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 1
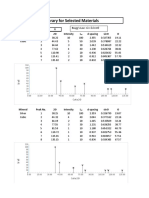
346 Electroplating, odizing and Metal Treatment Hand Book
Heavy zinc phosphate coatings ori steel exhibit a more platelike
structure than manganese phosphate . Two varieties are normall y
encountered . When maximum corrosion protection is desired, an iron
content is maintained in the phosphating solution and a crystal structure
is produced . The orientation is principally parallel to the metal surface
and the layered structure offers an excellent surface for the retention of
corrosion resistant oils and waxes.
When a heavy zinc phosphate is used to facilitate cold deformation
such as encountered in the drawing of wire and tubing, cold shaping,
cold extrusion, etc., the phosphating solution is operated under iron-free
conditions resulting in a crystal structure showing a high percentage of
crystals oriented vertically to the metal surface. These thin plates may be
lubricated by one of several methods, including lime, borax, specialized
soaps and oils. In some cases, the surfaces of the individual crystals may
be converted to a zinc . soap. These lubricated plates then provide a
multitude of slipping surfaces that promote the ease of cold deformation .
When a phosphate coating is used to promote paint bonding on
steel or zinc coated steel, a number of options are available to the finishing
engineer. A zinc phosphate coating may be desired for superior
performance in retarding the lateral creep of corrosion between the
organic coating and the metal originating at a scratch through the coating
to the metal. Typical crystal structures for a zinc phosphate formed from
the same solution on steel and galvanized steel. The coating on steel is
tightly bonded to the surface and the effective surface area of the
phosphate coating is considerably greater than the underlying metal.
The phenomenon assists in increasing the adhesion of the organic coating
which is further promoted by increasing the number of sites for mechanical
locking of the coating to the phosphate crystals.
A zinc phosphate coating on a galvanized surface shows a different
structure. In this case, the individual crystals are well-defined and are
distributed in a random orientation on the surface. Note that these
photomicrographs are at a higher magnification than that used for the
corrosion resistant or drawing phosphates. A fine grained phosphate is
desirable for a paint base in order to provide acceptable gloss with
minimum organic coating thickness.
A less expensive but still effective phosphate treatment to promote
paint bonding utilizes a so-called iron phosphate treatment on steel or
zinc rich surfaces. It consists of an extremely fine grained deposit of
mixed oxides and phosphates of iron. The crystal structure is so fine that
the coating is often classified as amorphous. When the same solution
used to produce this coating on steel is used to treat a galvanized surface.
Anda mungkin juga menyukai
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5795)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Overview of Iron and Steel MakingDokumen24 halamanOverview of Iron and Steel MakingAryan MishraBelum ada peringkat
- 1-Con TechDokumen37 halaman1-Con TechZELALEM100% (1)
- Volumetric Analysis QuestionsDokumen3 halamanVolumetric Analysis QuestionsJake Robinson100% (8)
- International Standard: Iron Oxide Pigments - Specifications and Methods of TestDokumen26 halamanInternational Standard: Iron Oxide Pigments - Specifications and Methods of TestHamid Damghani100% (1)
- Thermochemistry (Answers)Dokumen17 halamanThermochemistry (Answers)Venessa BlingBling ChunBelum ada peringkat
- Star Regulus AntimonyDokumen2 halamanStar Regulus Antimonytravellerfellow100% (2)
- By Pass System in The Dry ProcessDokumen34 halamanBy Pass System in The Dry Processfaheemqc100% (1)
- Process Cycles: Table 1: Phosp Hate Coatings F or Steel and IronDokumen1 halamanProcess Cycles: Table 1: Phosp Hate Coatings F or Steel and IronKimiaBelum ada peringkat
- Classif Ication of Process Steps: A. CleaningDokumen1 halamanClassif Ication of Process Steps: A. CleaningKimiaBelum ada peringkat
- Amorphous Phosphate Coatings On Aluminum SurfacesDokumen1 halamanAmorphous Phosphate Coatings On Aluminum SurfacesKimiaBelum ada peringkat
- Flow Processing Pembuatan Propellant Komposite PolybhutadineDokumen1 halamanFlow Processing Pembuatan Propellant Komposite PolybhutadineKimiaBelum ada peringkat
- OREAS 611 Certificate R1Dokumen22 halamanOREAS 611 Certificate R1juan ganoza garciaBelum ada peringkat
- Asmet Technical Introduction To Metallurgical Process Control Using ATAS Advanced Thermal Analysis System Eng v1 20062011Dokumen2 halamanAsmet Technical Introduction To Metallurgical Process Control Using ATAS Advanced Thermal Analysis System Eng v1 20062011fondershellBelum ada peringkat
- Lab Report 2Dokumen8 halamanLab Report 2Turan GuliyevBelum ada peringkat
- Cambridge O Level: Chemistry 5070/21 October/November 2022Dokumen12 halamanCambridge O Level: Chemistry 5070/21 October/November 2022jamshedBelum ada peringkat
- ISSFDokumen1 halamanISSFspibluBelum ada peringkat
- Cat Grease 5% MolyDokumen2 halamanCat Grease 5% MolycatoeraleifBelum ada peringkat
- Solution Manual For Information Technology Project Management 9th Edition Kathy SchwalbeDokumen34 halamanSolution Manual For Information Technology Project Management 9th Edition Kathy Schwalbemanequin.design8haoij100% (43)
- Eamcet Practice PapersDokumen54 halamanEamcet Practice PapersudaysrinivasBelum ada peringkat
- Edexcel GCE: Chemistry (Nuffield)Dokumen16 halamanEdexcel GCE: Chemistry (Nuffield)Winnie YewBelum ada peringkat
- Fat ManDokumen18 halamanFat ManYasir Khan0% (1)
- Industrial Report Template Engineering v1Dokumen99 halamanIndustrial Report Template Engineering v1Amirul HasanBelum ada peringkat
- Question Bank Final Year BDSDokumen19 halamanQuestion Bank Final Year BDSPoonam K JayaprakashBelum ada peringkat
- Ceramic Crystal StructuresDokumen18 halamanCeramic Crystal StructuresVincentBelum ada peringkat
- Problem SetDokumen1 halamanProblem SetIrish Blanza PonceBelum ada peringkat
- Directory of Operating Mines and QuarriesDokumen3 halamanDirectory of Operating Mines and QuarriesiaabaoBelum ada peringkat
- Limbaga - XRD LibraryDokumen77 halamanLimbaga - XRD LibraryEDISON LIMBAGABelum ada peringkat
- Indian Minerals Yearbook 2011: 50 EditionDokumen12 halamanIndian Minerals Yearbook 2011: 50 Editionmujib uddin siddiquiBelum ada peringkat
- Feeds and Speeds Titan Micro End MillsDokumen1 halamanFeeds and Speeds Titan Micro End MillsAngel Adan Llamas YañezBelum ada peringkat
- Peroxide Test: 6. PreparationDokumen1 halamanPeroxide Test: 6. PreparationEuderDiasBelum ada peringkat
- Production: Laboratory PreparationsDokumen2 halamanProduction: Laboratory PreparationsVinod NairBelum ada peringkat
- Tugas Bu MichaDokumen3 halamanTugas Bu MichaSiti RohmiyatiBelum ada peringkat
- Cambridge Secondary 1 CheckpointDokumen20 halamanCambridge Secondary 1 CheckpointAnisahBelum ada peringkat
- MSM R19 - Unit-2Dokumen28 halamanMSM R19 - Unit-2Madheswaran DharmapuriBelum ada peringkat