Answer On The Question #69582, Chemistry / Physical Chemistry
Diunggah oleh
Ahmad HarisDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Answer On The Question #69582, Chemistry / Physical Chemistry
Diunggah oleh
Ahmad HarisHak Cipta:
Format Tersedia
Answer
on the question #69582, Chemistry / Physical Chemistry
Question:
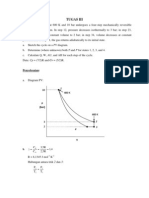
One mole of an ideal gas with Cp = (7/2) R and Cv = (5/2) R expands from P1= 8 bar & T1=
600K to P2 = 1bar by each of the following paths?
(1) Constant volume. (2) Constant temperature. (3) Adiabatically. Assuming mechanical
reversibility calculate W, Q, ∆ U and ∆ H for each process
Solution:
1) Constant volume process :
()
𝑊 = − 𝑝𝑑𝑉 = 0
(*
/)
𝑄 = 𝐶( 𝑑𝑇 = 𝐶( 𝑇0 − 𝑇1
/*
Let’s find the final temperature :
𝑝1 𝑝0
=
𝑇1 𝑇0
𝑝0 · 𝑇1 1 𝑏𝑎𝑟 · 600𝐾
𝑇0 = = = 75 𝐾
𝑝1 8 𝑏𝑎𝑟
Thus, heat is :
5
𝑄= · 8.314 · 75 − 600 = −10.91 𝑘𝐽
2
Change in internal energy is :
∆𝑈 = 𝑄 + 𝑊 = −10.91 𝑘𝐽
Change in enthalpy of ideal gas at constant volume is:
𝑅𝑇1
∆𝐻 = 𝑄 + 𝑉∆𝑃 = −10.91𝑘𝐽 + 𝑃 − 𝑃1
𝑃1 0
8.314𝐽/𝐾 · 600𝐾
= −10.91 𝑘𝐽 + (1𝑏𝑎𝑟 − 8𝑏𝑎𝑟)
8 𝑏𝑎𝑟
= −10.91 𝑘𝐽 − 4.36𝑘𝐽 = −15.27 𝑘𝐽
2) Constant temperature process :
Work done in the process :
()
𝑝0 𝐽 1
𝑊 = − 𝑝𝑑𝑉 = 𝑛𝑅𝑇𝑙𝑛 = 8.314 · 600𝐾 · ln = −10.37 𝑘𝐽
(* 𝑝1 𝐾 8
As for ideal gas, it’s internal energy change and enthalpy chage are zero as the temperature is
constant :
∆𝑈 = ∆𝐻 = 0
Then, heat change is :
𝑄 = ∆𝑈 − 𝑊 = 10.37 𝑘𝐽
3) Adiabatic process :
Change in heat is zero :
𝑄 = 0
Thus, internal energy is equal to work :
()
𝑛𝑅
∆𝑈 = 𝑊 = 𝑃𝑑𝑉 = 𝑇 − 𝑇1
(* 𝛾−1 0
QR T
where 𝛾 = = = 1.4.
QS U
Let’s find the final temperature :
VW1
𝑃0 V
𝑇0 = 𝑇1 = 331.2 𝐾
𝑃1
8.314 𝐽/𝐾
∆𝑈 = 𝑊 = 331.2 𝐾 − 600 𝐾 = −5.59 𝑘𝐽
1.4 − 1
Enthalpy of the process is :
7 8.314𝐽
∆𝐻 = 𝑛𝐶X ∆𝑇 = · · 331.2 𝐾 − 600 𝐾 = −7.82 𝑘𝐽
2 𝐾
Answer :
(1) 0 ; -10 .91 kJ ; -10.91 kJ ; -15.27 kJ
(2) -10.37 kJ ; 10.37 kJ ; 0 ; 0
(3) -5.59 kJ ; 0 ; -5.59 kJ ; -7.82 kJ
Answer provided by AssignmentExpert.com
Anda mungkin juga menyukai
- Tutorial Sheet 3 SolutionsDokumen10 halamanTutorial Sheet 3 SolutionsTENZIN WANGCHUKBelum ada peringkat
- LectureNotes - Thermal - Ch2,3,4Dokumen33 halamanLectureNotes - Thermal - Ch2,3,4Huy NguyenBelum ada peringkat
- APSC 252 Final Exam Formula SheetDokumen3 halamanAPSC 252 Final Exam Formula SheetYosloBelum ada peringkat
- Internal Combustion Engine Cycles - ExamplesDokumen16 halamanInternal Combustion Engine Cycles - Examplesmanjeet gajbhiye100% (1)
- The Entropy Change of Pure SubstancesDokumen5 halamanThe Entropy Change of Pure SubstancesAbd El-Razek AhmedBelum ada peringkat
- The Entropy Change of Pure SubstancesDokumen5 halamanThe Entropy Change of Pure SubstancesAbd El-Razek AhmedBelum ada peringkat
- First LawDokumen10 halamanFirst LawAhmed Al-ayatBelum ada peringkat
- Lecture Sheet 5 2023-11-26 13 - 56 - 19Dokumen8 halamanLecture Sheet 5 2023-11-26 13 - 56 - 19tawhidnabin.sylhetBelum ada peringkat
- Cc2 ThermodynamicsDokumen22 halamanCc2 Thermodynamicsmark anthony tutorBelum ada peringkat
- (x4) Problem 5 - 9 Multi Stage CompressionDokumen15 halaman(x4) Problem 5 - 9 Multi Stage CompressionLester Alfred M. OlasimanBelum ada peringkat
- Second Law of Thermodynamics in Terms of EntropyDokumen9 halamanSecond Law of Thermodynamics in Terms of Entropykhandaker raiyanBelum ada peringkat
- Week 4Dokumen28 halamanWeek 4Mohamed nasserBelum ada peringkat
- Lecture 03 First Law of Themodynamics - Closed SystemsDokumen34 halamanLecture 03 First Law of Themodynamics - Closed SystemsdinurjBelum ada peringkat
- MEE 515 - HVAC - Lecture 3Dokumen28 halamanMEE 515 - HVAC - Lecture 3Charbel KhouryBelum ada peringkat
- Lesson 6 - Thermodynamics I Basics and First LawDokumen34 halamanLesson 6 - Thermodynamics I Basics and First Lawjaydi.maat.02Belum ada peringkat
- CO3 Thermodynamics Sample Problem With AnswersDokumen6 halamanCO3 Thermodynamics Sample Problem With AnswersCarreon ChristianBelum ada peringkat
- Chapter 1 - TheRMODYNAMICS (Synchronous)Dokumen60 halamanChapter 1 - TheRMODYNAMICS (Synchronous)loyovaf500Belum ada peringkat
- Lab - Activity No. 6 - Rimbao, Alona Jane V.Dokumen5 halamanLab - Activity No. 6 - Rimbao, Alona Jane V.Alona Jane RimbaoBelum ada peringkat
- Technological University of The Philippines Ayala Boulevard, Ermita, Manila, Philippines College of Engineering Mechanical Engineering DepartmentDokumen11 halamanTechnological University of The Philippines Ayala Boulevard, Ermita, Manila, Philippines College of Engineering Mechanical Engineering DepartmentmarkBelum ada peringkat
- Cep HMT Abdullah FinalDokumen12 halamanCep HMT Abdullah FinalAnti Venom0% (1)
- Group10 Cpe 1 3Dokumen16 halamanGroup10 Cpe 1 3Joshua MistalBelum ada peringkat
- Tutorial Problems-Ch 5Dokumen36 halamanTutorial Problems-Ch 5nonstopforever9266Belum ada peringkat
- tutorialETD SolnsDokumen17 halamantutorialETD SolnsAkshay JaiwaliaBelum ada peringkat
- (Cont'd) The First Law of ThermodynamicsDokumen26 halaman(Cont'd) The First Law of Thermodynamicskevintjj35Belum ada peringkat
- Question # 01: AnswerDokumen9 halamanQuestion # 01: AnswerKhushnoodBelum ada peringkat
- Sheet 1 TutorialDokumen12 halamanSheet 1 TutorialEmptySilenceBelum ada peringkat
- 125452033-Thermodynamics D.J.Dunn PDFDokumen185 halaman125452033-Thermodynamics D.J.Dunn PDFkalite123Belum ada peringkat
- Tut6 SolnDokumen10 halamanTut6 SolnJaishuu ChoudharyBelum ada peringkat
- Chaters of ThermodynamicsDokumen106 halamanChaters of ThermodynamicsAzooBelum ada peringkat
- Experiment 2 (Value of J)Dokumen8 halamanExperiment 2 (Value of J)Tajnuba Nahar MriduBelum ada peringkat
- Che Thermodynamics: Dimensions and UnitsDokumen31 halamanChe Thermodynamics: Dimensions and UnitsRugi Vicente RubiBelum ada peringkat
- Specific Heats of An Ideal GasDokumen15 halamanSpecific Heats of An Ideal Gasch0k3 iiiBelum ada peringkat
- Second Law of Thermodynamics: T T Q QDokumen10 halamanSecond Law of Thermodynamics: T T Q Qnellai kumarBelum ada peringkat
- Otto Cycle: Internal Combustion Engine Is A Prime Mover inDokumen4 halamanOtto Cycle: Internal Combustion Engine Is A Prime Mover inJohn Michael CubarBelum ada peringkat
- Engg ThermodynamicsgfDokumen3 halamanEngg Thermodynamicsgfphysics a2Belum ada peringkat
- CET I 3.PVT Relationship 2021 Part 2Dokumen47 halamanCET I 3.PVT Relationship 2021 Part 2Dhruv AgarwalBelum ada peringkat
- CH 04Dokumen12 halamanCH 04hirenpatel_universalBelum ada peringkat
- Throttling PDFDokumen12 halamanThrottling PDFDiams AlifBelum ada peringkat
- Chapter 5 - ThermochemistryDokumen54 halamanChapter 5 - ThermochemistryVarunesh MauthialaganBelum ada peringkat
- Chapter 6Dokumen11 halamanChapter 6Jigs Sanares50% (2)
- Name: Reylan S. Javilo Grade and Section: 12-ENTROPY Gas Laws Exercise No. 1Dokumen3 halamanName: Reylan S. Javilo Grade and Section: 12-ENTROPY Gas Laws Exercise No. 1Jayson P. JalbunaBelum ada peringkat
- Reversible and Irreversible ProcesesDokumen12 halamanReversible and Irreversible ProcesesFarouk BassaBelum ada peringkat
- TDY1 - Les 3 EngDokumen28 halamanTDY1 - Les 3 EngBrandon GesterkampBelum ada peringkat
- محاضرة الكيمياء الفيزياوية 4Dokumen3 halamanمحاضرة الكيمياء الفيزياوية 4Fuji 57Belum ada peringkat
- 1ST Law of ThermodynamicsDokumen19 halaman1ST Law of ThermodynamicsZamanoden D. UndaBelum ada peringkat
- Kelompok 1 TermodinamikaDokumen38 halamanKelompok 1 TermodinamikaArdina Ayu WulandariBelum ada peringkat
- Reactors Design - 1603888, 1599618 I 1603509Dokumen9 halamanReactors Design - 1603888, 1599618 I 1603509saramartori.2002Belum ada peringkat
- 2018-6-14 Advanced Environmental ProtectionDokumen10 halaman2018-6-14 Advanced Environmental ProtectionSoveasna ChanBelum ada peringkat
- Guerrero, Mary Justine A. - ChE 192 U - Heat Exchanger Design ProblemDokumen15 halamanGuerrero, Mary Justine A. - ChE 192 U - Heat Exchanger Design ProblemJustine GuerreroBelum ada peringkat
- Summary of Lecture 21, 22, 23 & 24: ThermometersDokumen4 halamanSummary of Lecture 21, 22, 23 & 24: ThermometersStevenChandraBelum ada peringkat
- ThermodynamicsDokumen12 halamanThermodynamicsKira ToBelum ada peringkat
- Module 3 - Second Law of Thermodynamics - 1319551938Dokumen12 halamanModule 3 - Second Law of Thermodynamics - 1319551938Lei LopezBelum ada peringkat
- Assignment in ThermochemistryDokumen1 halamanAssignment in Thermochemistryanon_9154496090% (1)
- PART 1 Solved Problems Cooling TowerDokumen11 halamanPART 1 Solved Problems Cooling TowerPETER PENBelum ada peringkat
- Group 7 FixDokumen30 halamanGroup 7 Fixyuni fitriaBelum ada peringkat
- S5 2020 2 AulaDokumen46 halamanS5 2020 2 AulaAlterno PuentesBelum ada peringkat
- THW No Longer Accept TheDokumen1 halamanTHW No Longer Accept TheAhmad HarisBelum ada peringkat
- Liquid LiquidMixinginStirredVesselsDokumen4 halamanLiquid LiquidMixinginStirredVesselsAhmad HarisBelum ada peringkat
- MSDS Essential Oil CitronellaDokumen4 halamanMSDS Essential Oil CitronellaMasneli MasriBelum ada peringkat
- Jawaban Tugas IIIDokumen6 halamanJawaban Tugas IIIAmira NatasyaBelum ada peringkat
- Efek TyndalDokumen1 halamanEfek TyndalAhmad HarisBelum ada peringkat
- B. Contoh Dialog Bahasa Inggris Expressing Offering HelpDokumen2 halamanB. Contoh Dialog Bahasa Inggris Expressing Offering HelpAhmad HarisBelum ada peringkat
- 1-S2.0-S0169433213006843-Main SurfactantDokumen6 halaman1-S2.0-S0169433213006843-Main SurfactantAhmad HarisBelum ada peringkat
- Appendik1 AccDokumen1 halamanAppendik1 AccAhmad HarisBelum ada peringkat
- Appendiks FixDokumen2 halamanAppendiks FixAhmad HarisBelum ada peringkat
- Tranferencias Segun IEEE 446Dokumen8 halamanTranferencias Segun IEEE 446Jorge Ignacio MVBelum ada peringkat
- JEE Main 2019 Detailed Analysis April Attempt Shift - 1 (08th April, 2019)Dokumen7 halamanJEE Main 2019 Detailed Analysis April Attempt Shift - 1 (08th April, 2019)Resonance Eduventures100% (1)
- Symmetrical Components 2Dokumen15 halamanSymmetrical Components 2CaribBelum ada peringkat
- StatisticsDokumen34 halamanStatisticsPoth BaliyanBelum ada peringkat
- Eng. Khalid Footing-DesignDokumen60 halamanEng. Khalid Footing-Designjunaid112Belum ada peringkat
- GDT-Related Actual Mating EnvelopeDokumen10 halamanGDT-Related Actual Mating EnvelopeManoj Kumar MurmuBelum ada peringkat
- An Exploration of The Physics Behind Rail GunsDokumen7 halamanAn Exploration of The Physics Behind Rail GunsmegustalazorraBelum ada peringkat
- Most Scoring Concepts: For Neet 2022Dokumen48 halamanMost Scoring Concepts: For Neet 2022Rajesh K Singh0% (1)
- PCEG 403 Lab No. 1 Title: Simulation of Single Phase Half Wave Converter DC DriveDokumen5 halamanPCEG 403 Lab No. 1 Title: Simulation of Single Phase Half Wave Converter DC DriveJanup PokharelBelum ada peringkat
- Phy1 11 - 12 Q1 0603 PF FDDokumen68 halamanPhy1 11 - 12 Q1 0603 PF FDhiroBelum ada peringkat
- Rewind Motor BrushlessDokumen11 halamanRewind Motor Brushlesssharingiscaring69Belum ada peringkat
- Powder DiffractionDokumen9 halamanPowder DiffractionGary TrumpBelum ada peringkat
- Sensors: Gyroscope Technology and Applications: A Review in The Industrial PerspectiveDokumen22 halamanSensors: Gyroscope Technology and Applications: A Review in The Industrial PerspectiveVijay GorfadBelum ada peringkat
- Philip L. Bowers - Lectures On Quantum Mechanics - A Primer For Mathematicians (2020, Cambridge University Press)Dokumen584 halamanPhilip L. Bowers - Lectures On Quantum Mechanics - A Primer For Mathematicians (2020, Cambridge University Press)Rodrigo Osorio Guerra100% (1)
- The SunDokumen16 halamanThe Sun3XTR3M3 ᜰ꙰ꦿ NJRBelum ada peringkat
- MOT NewDokumen37 halamanMOT Newdsw27Belum ada peringkat
- Lecture Notes PDFDokumen46 halamanLecture Notes PDFManex ManBelum ada peringkat
- MAT 217 Lecture 4 PDFDokumen3 halamanMAT 217 Lecture 4 PDFCarlo KaramBelum ada peringkat
- Electrostatics 11: Electric ChargeDokumen69 halamanElectrostatics 11: Electric ChargeManas kumarBelum ada peringkat
- Textbook of ElectrotherapyDokumen345 halamanTextbook of ElectrotherapyAlice Teodorescu100% (19)
- Lecture15 Pile GroupsDokumen48 halamanLecture15 Pile Groupsviraj100% (1)
- Sommerfeld Arnold PDFDokumen211 halamanSommerfeld Arnold PDFfahrul3xBelum ada peringkat
- AscherH The Dynamics and Vol.1-1953Dokumen6 halamanAscherH The Dynamics and Vol.1-1953jvmazloum7Belum ada peringkat
- Level 2 Ques (1) RTDokumen34 halamanLevel 2 Ques (1) RTVishal Sharma100% (1)
- Measuring Magnetic FieldDokumen6 halamanMeasuring Magnetic FieldAleesya RinaraBelum ada peringkat
- Tutorial 4: Magnetism: KML - Tutorial SP025 PhysicsDokumen3 halamanTutorial 4: Magnetism: KML - Tutorial SP025 PhysicsjessycaBelum ada peringkat
- Shaping MachineDokumen14 halamanShaping MachineMohammad Javed IqbalBelum ada peringkat
- Dosimetry Materials BrochureDokumen4 halamanDosimetry Materials BrochureVictor Manuel Suyco CasaperaltaBelum ada peringkat
- Zno For Arsenic SensingDokumen8 halamanZno For Arsenic SensingAamir AhmedBelum ada peringkat
- Lesson 3.3 Inside An AtomDokumen42 halamanLesson 3.3 Inside An AtomReign CallosBelum ada peringkat