Chemical Elements Crossword 1
Diunggah oleh
David Lockart0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
681 tayangan2 halamanUnreactive, gaseous element that is a product of the nuclear reaction (fusion) of hydrogen atoms. Used in blimps because of its low density. Highly reactive metal used in manufacture of synthetic rubber and drugs.
Deskripsi Asli:
Hak Cipta
© Attribution Non-Commercial (BY-NC)
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniUnreactive, gaseous element that is a product of the nuclear reaction (fusion) of hydrogen atoms. Used in blimps because of its low density. Highly reactive metal used in manufacture of synthetic rubber and drugs.
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
681 tayangan2 halamanChemical Elements Crossword 1
Diunggah oleh
David LockartUnreactive, gaseous element that is a product of the nuclear reaction (fusion) of hydrogen atoms. Used in blimps because of its low density. Highly reactive metal used in manufacture of synthetic rubber and drugs.
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 2
Chemical Elements Crossword
Chemical Elements Crossword.doc
Down:
1. An unreactive, gaseous element that is a product of the nuclear reaction (fusion) of hydrogen atoms. This reaction occurred at the
beginning of time and occurs today in stars such as our sun. The second most abundant element in the universe, it is quite rare on
Earth. Small concentrations are found in some gas natural deposits. It is used in blimps because of its low density. (Only hydrogen,
which is highly flammable, has a lower density). It is also used in cryogenic (low-temperature) work because it can be compressed to
a liquid that has a temperature of -269° C.
2. A reactive, metallic element. Its compounds are used as a medical "cocktail" to outline the stomach and intestines for X-ray
examination. Its compounds also give green colors to fireworks.
4. A highly reactive metal. It is used in the manufacture synthetic rubber and drugs. Recently one of its compounds has been used to
successfully treat a certain type of mental illness. It finds limited use in nuclear bombs.
6. A widely distributed nonmetal which is never found in its free, elemental state. It is an essential component in all cell protoplasm,
DNA, and various animal tissues and bones. It is also one of the three main elements in fertilizers.
9. An unreactive gas. In the comic book world, a mineral containing this element could weaken Superman. In the real world, a
radioactive form of this element is a byproduct of most nuclear explosions and its presence in the atmosphere can indicate which
nations are testing nuclear weapons.
10. Reactive metal with high melting point. It is used in the manufacture of rocket nose cones because it is very strong for its low density.
12. A reactive, silver-white metal that is second in abundance to sodium in ocean water. Due to its low density and high strength, its
alloys are often used for structural purposes in the transportation industry, as in "mag" wheels. It is also used in photo flash cubes,
fireworks and incendiary bombs because it ignites very readily. Some of its compounds, such as Epsom salt and milk of magnesia,
serve medicinal purposes.
14. A component of all living matter and fossil fuels; the black material on a charred candlewick.
18. Nicknamed quicksilver, it is the only metal which is a liquid at room temperature. It is used in thermometers because it expands
significantly and regularly when heated. Its high density makes it a practical substance to use in barometers. (Note: A barometer is
used to measure atmospheric air pressure). It is a toxic "heavy" metal.
19. The lightest and most abundant element; the fuel of the universe. It is believed that all other elements were originally formed from a
series of stellar nuclear reactions beginning with this one. It is found in numerous compounds such as water and most compounds
containing carbon.
20. A highly reactive metal of low density. It is one of the three main elements found in fertilizer. Its compounds are quite similar to those
of sodium, though typically more expensive.
22. A soft, dense metal used in bullets and car batteries. It was once used extensively both in plumbing and in paints. Concern over its
biological effects has caused a ban on its use for these purposes. It is being slowly phased out as a gasoline additive for the same
reason.
25. With the highest melting point of any pure element, it is the filament in ordinary (incandescent) light bulbs. Its one-letter symbol
comes from the name wolfram.
27. A metallic element. It is added to steel to increase its strength.
28. A metallic element which serves as the negative pole (electrode) in the common flashlight battery. It is used to plate a protective film
on iron objects (as in galvanized buckets). Melted with copper it becomes brass.
29. A metal that is used to make stainless steel. Combined with nickel, it forms nichrome wire which is used in toasters and other devices
where high electrical resistance (to produce heat) is desired.
30. This metal has a relatively low melting point and it is used in fire detection and extinguishing devices as well as in electrical fuses.
31. The most chemically reactive metal. Though it is quite rare, it is used in some photoelectric cells and in atomic clocks, which have far
greater accuracy than our mechanical or electric clocks.
33. A reddish, lustrous, ductile, malleable metal that occurs in nature in both free and combined states. It forms the body of the Statue of
Liberty. Other uses include electrical wiring, pennies, and decorative objects.
34. A magnetic, metallic element used extensively for structural purposes. Outdoor stair railings at home may be made of this element.
38. A yellow, non-reactive, metallic element that has been highly valued for its beauty and durability since ancient times.
39. A metallic element that is used as a corrosion-resistant coating on the inside of cans used for packaging food, oil, and other
substances.
Across
3. A highly reactive, gaseous non-metal. Its compounds are added to some toothpastes and many urban water supplies to prevent tooth
decay.
5. A reactive metal whose compounds make up limestone, chalk, cement, and the bones and teeth of animals. Milk is a good nutritional
source of this element.
7. An expensive, silver-white metal used in jewelry. It is also used in some industrial processes to speed up chemical reactions.
8. A solid purple -black nonmetal which changes to deep purple gas upon heating. An alcohol solution of this element serves as an
effective skin disinfectant. A compound of the element is added to sodium chloride (table salt) to prevent goiter.
10. Used in borosilicate (pyrex) glass, Boraxo soap, drill bits, and control rods in nuclear power plants.
11. One of the three magnetic elements, this metal is used in 5-cent pieces and other coins, in electroplanting, and in nichrome wire.
13. The most abundant metal in the earth's crust, this silver-white element is characterized by its low density, resistance to corrosion, and
high strength. It is used for a variety of structural purposes, such as in airplanes, boats, and cars.
14. A hard, magnetic metal used in the production of steel. A radioactive form of this element is used in cancer treatment.
15. A silver-white, lustrous, radioactive metal. Used as fuel in nuclear power plants and in atomic warheads.
16. An unreactive, gaseous element used in advertising signs for the bright reddish-orange glow it produces when an electric current is
passed through it.
17. A yellow nonmetal that occurs in both the free and combined states. It is used in making match tips, gunpowder, and vulcanized
rubber. Its presence in coal leads to acid (sulfuric acid) rain if it is not removed before the coal is burned.
21. This metal is the best conductor of heat and electricity. Its scarcity prevents it from being commonly used for such purposes. It was
used extensively in the past in the manufacture of coins, but has become too expensive. It is used today for fine eating utensils and
decorative objects. Some of its compounds are sensitive enough to light to be used in photographic film.
23. A gaseous nonmetal, the most abundant element on earth. It makes up some 21% of the earth's atmosphere and is essential to most
forms of life for the conversion of food into energy.
24. The second most abundant element in the Earth's crust. It is the principal component of sand and quartz and finds use in the solar
cells, computer chips, caulking materials, and abrasives.
26. A metallic element used in control rods in nuclear power plants and in Ni-Cad rechargeable batteries.
30. A red, highly reactive, fuming liquid with a foul smell. It finds limited use as a disinfectant. One of its compounds is a component of
leaded gasoline.
32. An odorless, colorless, unreactive gas used in most incandescent lightbulbs.
35. An element whose symbol comes from its Latin name. It is used with lead in car batteries.
36. A soft, highly reactive metal whose compounds include table salt, lye, and baking soda.
37. A gaseous nonmetal that makes up 78% of our atmosphere. Its compounds are important components of protein, fertilizers, and many
explosives.
40. A highly reactive, greenish-yellow gas used a bleach, as a disinfectant (in the purification of water), and as a poison gas.
Anda mungkin juga menyukai
- A Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksDari EverandA Closer Look at Silicon - Chemistry Book for Elementary | Children's Chemistry BooksBelum ada peringkat
- 10th Class Project Works - 8Dokumen5 halaman10th Class Project Works - 8Rama mohan palammagariBelum ada peringkat
- SMK Puteri Seremban: Chemistry ProjectDokumen28 halamanSMK Puteri Seremban: Chemistry ProjectHaemaavadhaanaBelum ada peringkat
- Angel CondensadaDokumen6 halamanAngel CondensadaAsnipah Dilaos MustapahBelum ada peringkat
- A MetalDokumen4 halamanA MetalThanigesan MahalingamBelum ada peringkat
- Class 8 Metals and Non - MetalsDokumen53 halamanClass 8 Metals and Non - Metalsmanjulata.mohanty85Belum ada peringkat
- Metals & Non-Metals ClassificationDokumen4 halamanMetals & Non-Metals ClassificationHitesh Alwadhi0% (1)
- Extractive Metallurgy 1: Basic Thermodynamics and KineticsDari EverandExtractive Metallurgy 1: Basic Thermodynamics and KineticsBelum ada peringkat
- Metals and Non-Metals Notes Provided by TeacherDokumen7 halamanMetals and Non-Metals Notes Provided by TeacherAdeeba Raheel QureshiBelum ada peringkat
- How Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksDari EverandHow Is Mercury Used Today? Chemistry Book for Kids 9-12 | Children's Chemistry BooksBelum ada peringkat
- Study of Constituents of An AlloyDokumen18 halamanStudy of Constituents of An AlloyMohammed Anis RahmanBelum ada peringkat
- General Chemistry Script and FlowDokumen7 halamanGeneral Chemistry Script and FlowdecastroghislaneBelum ada peringkat
- Chemi Presentation 2Dokumen25 halamanChemi Presentation 2teddyBelum ada peringkat
- LECTURE No. 7. LOW MELTING CRYOGENIC ALLOYS-MARAGING-SUPERALLOYSDokumen8 halamanLECTURE No. 7. LOW MELTING CRYOGENIC ALLOYS-MARAGING-SUPERALLOYSBizuayehu Tadesse AzeneBelum ada peringkat
- 9E Reactions of Metals andDokumen18 halaman9E Reactions of Metals and陳信羽Belum ada peringkat
- Definition of Elements 1-20Dokumen2 halamanDefinition of Elements 1-20War ThunderBelum ada peringkat
- Elements of Modern Style ArticleDokumen5 halamanElements of Modern Style ArticlemamazookeeprBelum ada peringkat
- Properties and Uses of Metals, Nonmetals and MineralsDokumen14 halamanProperties and Uses of Metals, Nonmetals and Mineralswama ojhaBelum ada peringkat
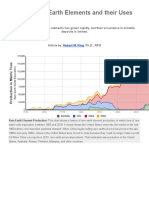
- REE Rare EarthsDokumen11 halamanREE Rare EarthsRicardo JesusBelum ada peringkat
- Interactive Textbook 5 2Dokumen9 halamanInteractive Textbook 5 2api-240094705Belum ada peringkat
- Native Elements Group - Properties and UsesDokumen5 halamanNative Elements Group - Properties and UsesBroadsageBelum ada peringkat
- Elements Matching GameDokumen4 halamanElements Matching GameMoath AlsaidBelum ada peringkat
- 7th Chemistry, L-6, Metals and Non-MetalsDokumen4 halaman7th Chemistry, L-6, Metals and Non-MetalsDEEPAK JAINBelum ada peringkat
- Alkali Metal - Britannica Online EncyclopediaDokumen10 halamanAlkali Metal - Britannica Online EncyclopediaAMRENDRA KUMARBelum ada peringkat
- General Properties of Alkali MetalsDokumen8 halamanGeneral Properties of Alkali MetalsKhawaja Rehan AhmedBelum ada peringkat
- Electroplating Plastic ProcessDokumen7 halamanElectroplating Plastic ProcessImpian SiberBelum ada peringkat
- Chemistry ProjectDokumen13 halamanChemistry ProjectPavizham Rajesh kBelum ada peringkat
- Mineralsandtheiruses Part 1nativeelements PDFDokumen10 halamanMineralsandtheiruses Part 1nativeelements PDFjosueBelum ada peringkat
- Pharma Chem Mid TermsDokumen10 halamanPharma Chem Mid Terms2241689Belum ada peringkat
- Metals Summary Notes GuideDokumen7 halamanMetals Summary Notes GuideRohanBelum ada peringkat
- Pictures of Elements: Hydrogen - Element 1Dokumen33 halamanPictures of Elements: Hydrogen - Element 1Praveen JoshiBelum ada peringkat
- Group 14 (IVA) Elements - PHPDokumen15 halamanGroup 14 (IVA) Elements - PHPtrouton7Belum ada peringkat
- Metals: Physical Properties of MetalDokumen6 halamanMetals: Physical Properties of MetalAllen Jierqs SanchezBelum ada peringkat
- Ga Element Guide - Properties of Gallium, Beryllium & RutheniumDokumen4 halamanGa Element Guide - Properties of Gallium, Beryllium & Rutheniumavipsa ghoshBelum ada peringkat
- Solutions for a Cleaner, Greener Planet: Environmental ChemistryDari EverandSolutions for a Cleaner, Greener Planet: Environmental ChemistryBelum ada peringkat
- Pure substances notes -2Dokumen5 halamanPure substances notes -2vanshikaa.2613Belum ada peringkat
- Materials Metals and Non-MetalsDokumen11 halamanMaterials Metals and Non-MetalsSrishti SangamBelum ada peringkat
- IGCSE Chemistry A - Notes Chapter 9 - The Periodic TableDokumen28 halamanIGCSE Chemistry A - Notes Chapter 9 - The Periodic TableShadman RahmanBelum ada peringkat
- Book E and CDokumen37 halamanBook E and CKyla Marie HernandezBelum ada peringkat
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookDari EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookBelum ada peringkat
- Melane L. Ayuyu CHEM 115: HydrogenDokumen10 halamanMelane L. Ayuyu CHEM 115: HydrogenMelaneBelum ada peringkat
- A New Look at The Periodic TableDokumen11 halamanA New Look at The Periodic TableJ. GirotoBelum ada peringkat
- Section 2Dokumen11 halamanSection 2Jimmy gogoBelum ada peringkat
- Copper Design ManualDokumen22 halamanCopper Design Manualamr_scorpion_engBelum ada peringkat
- Business of Rare Earth ElementsDokumen6 halamanBusiness of Rare Earth ElementsAnanthBelum ada peringkat
- Noble GasesDokumen25 halamanNoble GasesandreasaryasatyaBelum ada peringkat
- Science Test BookDokumen3 halamanScience Test Bookayush sonar100% (1)
- Material de 20 ENGENHARI 20 ECONOMICA - Odt - 1Dokumen15 halamanMaterial de 20 ENGENHARI 20 ECONOMICA - Odt - 1Alcino SebastiãoBelum ada peringkat
- Nickel, gold, copper and other metals comparedDokumen5 halamanNickel, gold, copper and other metals comparedRofilR.AlbaoBelum ada peringkat
- ElectrorafinationDokumen13 halamanElectrorafinationedisanilaBelum ada peringkat
- Oxidation Reduction and Metal-04Dokumen4 halamanOxidation Reduction and Metal-04SimantoPreeomBelum ada peringkat
- GermaniumDokumen2 halamanGermaniumMiles Heman-AckahBelum ada peringkat
- Week 7 Periodic Table of Elements LECTURE G8Dokumen37 halamanWeek 7 Periodic Table of Elements LECTURE G8sibugzaldyBelum ada peringkat
- The Periodic Table CDokumen177 halamanThe Periodic Table Cmarius1966Belum ada peringkat
- Metal and NonmetalDokumen26 halamanMetal and NonmetalSudhanshu Sekhar PandaBelum ada peringkat
- Cobalt-based superalloys key use in turbinesDokumen7 halamanCobalt-based superalloys key use in turbinesAlexandra ChiengBelum ada peringkat
- Importance of Periodic TableDokumen3 halamanImportance of Periodic TableMARASINGHE ARACHCHIGE LAKSHAN PATHUM� KUMARABelum ada peringkat
- Classification of MaterialsDokumen113 halamanClassification of Materialsabhinavgiri17Belum ada peringkat
- 3.1 Chemistry of Metals FULLDokumen41 halaman3.1 Chemistry of Metals FULLRenejhon MaquilanBelum ada peringkat
- Stuff To Remember (Chem)Dokumen17 halamanStuff To Remember (Chem)Tamilore SobowaleBelum ada peringkat
- WS 18 FinalDokumen4 halamanWS 18 FinalLyra GurimbaoBelum ada peringkat
- Concrete Mix Design M-40 (RCC) Grade: Name of Work Client Authority Engineer ContractorDokumen34 halamanConcrete Mix Design M-40 (RCC) Grade: Name of Work Client Authority Engineer ContractorAshok amlapureBelum ada peringkat
- Experimental Study On Light Weight Concrete Using Coconut Shell and Fly AshDokumen9 halamanExperimental Study On Light Weight Concrete Using Coconut Shell and Fly AshIJRASETPublicationsBelum ada peringkat
- Solution To Problem Set No 2 ThermodynamicsDokumen4 halamanSolution To Problem Set No 2 ThermodynamicsMark Augusto V. AgusBelum ada peringkat
- Technical Handbook Air CurtainsDokumen13 halamanTechnical Handbook Air Curtainsaca111111Belum ada peringkat
- Fundamentals of Soil StabilizationDokumen25 halamanFundamentals of Soil StabilizationFrancheska BeltranBelum ada peringkat
- River Morphology - Garde - IndiaDokumen502 halamanRiver Morphology - Garde - Indiaburreiro100% (4)
- Indoor Environmental QualityDokumen29 halamanIndoor Environmental QualitykaigaBelum ada peringkat
- The 48 Laws of Power-5Dokumen10 halamanThe 48 Laws of Power-5probiggy007Belum ada peringkat
- BTB PS CR LabDokumen7 halamanBTB PS CR LabKathy XiangBelum ada peringkat
- Chapter 4 Study Guide The Periodic Table Section 1Dokumen2 halamanChapter 4 Study Guide The Periodic Table Section 1SyfensBelum ada peringkat
- Copia Controlada: ESP: 03101.T136Dokumen1 halamanCopia Controlada: ESP: 03101.T136Samuel MuñozBelum ada peringkat
- SPE Activities Workshop GuideDokumen33 halamanSPE Activities Workshop GuideEvan FNHBelum ada peringkat
- Evaluating Nuclear Energy Risks vs Fossil FuelsDokumen3 halamanEvaluating Nuclear Energy Risks vs Fossil FuelsShubham SinghBelum ada peringkat
- Biodiversity Report 3Dokumen3 halamanBiodiversity Report 3SAILAKSHMI CBelum ada peringkat
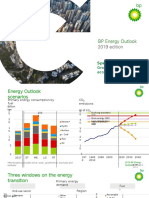
- BP Energy Outlook 2019 Presentation SlidesDokumen29 halamanBP Energy Outlook 2019 Presentation SlidesAJBelum ada peringkat
- Energy - Solar - Water Heating System Designs - (Ebook Construction Building How To Diy) (Tec@Nz)Dokumen8 halamanEnergy - Solar - Water Heating System Designs - (Ebook Construction Building How To Diy) (Tec@Nz)ABELWALIDBelum ada peringkat
- Boiler Inspection MaintenanceDokumen5 halamanBoiler Inspection Maintenanceskthen72Belum ada peringkat
- Environmental Impact Assessment of Ting Ting Hydroelectric Project, SikkimDokumen18 halamanEnvironmental Impact Assessment of Ting Ting Hydroelectric Project, SikkimShreya MalikBelum ada peringkat
- Newtons Laws of MotionDokumen14 halamanNewtons Laws of MotionMakayla SalmonBelum ada peringkat
- ENERGY SAVING STEEL PRODUCTION DECARBONIZATION EUDokumen7 halamanENERGY SAVING STEEL PRODUCTION DECARBONIZATION EUMOKKA AKHIL KUMAR RGUKT BasarBelum ada peringkat
- A Novel Approach to Standard Modeling and Simulation of MicrogridsDokumen22 halamanA Novel Approach to Standard Modeling and Simulation of Microgridsraj100% (1)
- Energies 15 03452Dokumen20 halamanEnergies 15 03452Shreesha KumarBelum ada peringkat
- Comparison Static UPS - Rotary UPSDokumen1 halamanComparison Static UPS - Rotary UPSdelmar02Belum ada peringkat
- Tutorial 3 Heat Exchanger PDFDokumen22 halamanTutorial 3 Heat Exchanger PDFBipin GiriBelum ada peringkat
- Class 10 Chapter 5 Minerals and Energy Resources-SignedDokumen8 halamanClass 10 Chapter 5 Minerals and Energy Resources-SignedK SHAKTHIBelum ada peringkat
- Chapter 9 HydrogenDokumen19 halamanChapter 9 HydrogenYash PlayBelum ada peringkat
- METAL ORGANIC FRAMEWORKS (MOFs)Dokumen8 halamanMETAL ORGANIC FRAMEWORKS (MOFs)FabianCcahuanaAymaBelum ada peringkat
- Reaction of Beam: Roll No. Fy20H Y68Dokumen4 halamanReaction of Beam: Roll No. Fy20H Y68Radhika GaikwadBelum ada peringkat
- 03 - Andianto - Bayu PERTAMINA BIOGAS FORUMDokumen12 halaman03 - Andianto - Bayu PERTAMINA BIOGAS FORUMwangintanBelum ada peringkat