3'-Thiol-Modifier C6 S-S CPG
Diunggah oleh
AlleleBiotechJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
3'-Thiol-Modifier C6 S-S CPG
Diunggah oleh
AlleleBiotechHak Cipta:
Format Tersedia
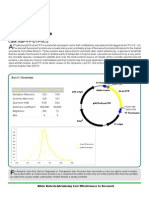
3’ Thiol, 3’-Thiol-modifier C6
S-S CPG
Box 1 | Product List
Beaucage SL. Strategies in the preparation of DNA oligonucleotide
3’ Thiol-Modifier C6 S-S CPG arrays for diagnostic applications. Curr Medicinal Chem, 8, 1213-1244.
(2001)
Cat. No. Amount
AHP-CH-TC60100 100 mg Gorodetsky AA, Barton JK. Electrochemistry using self-assembled DNA
monolayers on highly oriented pyrolytic graphite. Langmuir, 22, 7917-
AHP-CH-TC61000 1000 mg
7922. (2006)
Liu RH, Coty WA, Reed MW, Gust G. Electrochemical detection-based
DNA microarrays, IVD Tech, 31. (2008)
Name: 3’-Thiol-modifier C6 S-S CPG
Chemical Name: 1-O-Dimethoxytrityl-hexyl-disulfide,1’-succi- Umek RM, Lin SW, Vielmetter J, Terbrueggen RH, Yu CJ, Kayyem JF,
noyl-long chain alkylamino-CPG Irvine B, Yowanto H, Blackburn GF, Farkas DH, Chen YP. Electronic
Formula: N/A detection of nucleic acids: a versatile platform for molecular diagnostics.
J Mol Diagn, 3, 74-84. (2001)
Use: 3’-Thiol-modifier C6 S-S CPG is used to label a thiol group Lockett, MR, Phillips, MF, Jarecki, JL, Peelen, D, Smith LM. A tetrafluo-
at the 3’ end of oligonucleotides. rophenol activated ester self-assembled monolayer for the immobiliza-
Purity: 95%+ tion of amine-modified oligonucleotides. Langmuir, 24 (1), 69-75. (2008)
Diluent: Anhydrous Acetonitrile
Stability in Solution: N/A Wei F, Wang J, Liao W, Zimmermann BG, Wong DT, Ho CM. Electro-
chemical detection of low-copy number salivary RNA based on specific
Background: signal amplification with a hairpin probe. Nucleic Acids Res, 36(11), e65.
(2008)
Thiol modifications are important in the production of diagnos-
tic probes. They are use in DNA synthesizers to functionalize Frutos AG, Brockman JM, Corn RM. Reversible protection and reactive
oligonucleotides. The terminal thiol group allows attachment of patterning of amine and hydroxyl-terminated self-assembled monolay-
an oligo to various ligands, such as biotin, alkaline phosphatase ers on gold surfaces for the fabrication of biopolymer arrays. Langmuir,
or fluorescent dyes. Via an iodoacetamide or maleimide link- 16, 2192-2197. (2000)
age, it allows for the attachment of an oligo to a solid surface.
Thiol modification can be placed at either the 5’ or 3’ end of the Liepold P, Kratzmüller T, Persike N, Bandilla M, Hinz M, Wieder H,
oligonucleotide. For introduction of a thiol group at the 3’-end, Hillebrandt H, Ferrer E, Hartwich G. Electrically detected displacement
thiol-modifier C6 S-S CPG is available. To introduce a thiol assay (EDDA): a practical approach to nucleic acid testing in clinical or
group at the 5’ end, either 5’-thiol-modifier-C6 or 5’-thiol modi- medical diagnosis. Anal Bioanal Chem, 391, 1759-72. (2009)
fier C6 S-S may be used.
Li Z, Jin R, Mirkin CA, Letsinger RL. Multiple thiol-anchor capped DNA-
Coupling: All oxidation steps should use 0.02M Iodine solution gold nanoparticle conjugates. Nucleic Acids Res., 30, 1558-62. (2002)
to avoid oxidative cleavage of the disulfide linkage.
Demers LM, Mirkin CA, Mucic RC, Reynolds RA III, Letsinger RL.
Deprotection: After proceeding with normal deprotection, the Fluorescence-based method for determining the surface coverage and
disulfide can be cleaved at room temperature in 30 minutes with hybridization efficiency of thiol-capped oligonucleotides bound to gold
a buffered 100 mM DTT pH 8.3 - 8.5 solution. It is imporant to thin films and nanoparticles. Anal Chem, 72, 5535–5541. (2000)
completely remove the reducing agent before using the oligo in
a coupling reaction. Letsinger RL, Elghanian R, Viswanadham G, Mirkin CA. Use of a ster-
oid cyclic disulfide anchor in constructing gold nanoparticle-oligonucleo-
Storage: Freezer storage, -10 to -30°C, dry, dark area tide conjugates,” Bioconjugate Chem, 11(2), 289–291. (2000)
Labeled Oligo Storage: Store labeled oligo in the dark, either
dry or in a neutral aqueous media at -20°C. Do not store crude
fluorescently labeled oligonucleotides using ammonia.
MSDS: www.allelebiotech.com
Reference:
Phillips MF, Lockett MR, Rodesch MJ, Shortreed MR, Cerrina F, Smith
LM. In situ oligonucleotide synthesis on carbon materials: stable sub-
strates for microarray fabrication. Nucleic Acids Res, 36, e7. (2008)
Anda mungkin juga menyukai
- Articulo Quimica Medicinal 3Dokumen16 halamanArticulo Quimica Medicinal 3Jose Antonio Espinosa TorresBelum ada peringkat
- J. Biol. Chem.-1988-Hodges-11768-75Dokumen8 halamanJ. Biol. Chem.-1988-Hodges-11768-75Inggrid MadaniBelum ada peringkat
- MBP 2 DimageDokumen7 halamanMBP 2 DimageDolphingBelum ada peringkat
- NIH Public Access: Author ManuscriptDokumen27 halamanNIH Public Access: Author ManuscriptChristian AlvisBelum ada peringkat
- Biopolym - Cell 2018 34 5 374 enDokumen13 halamanBiopolym - Cell 2018 34 5 374 enАнна ШаповаловаBelum ada peringkat
- 7265Dokumen8 halaman7265DiogomussumBelum ada peringkat
- FA and TAGS NamesDokumen19 halamanFA and TAGS NamesIryna BonBelum ada peringkat
- 1985 Biochem Molecular Structure of The b3 Adrenergic ReceptorDokumen7 halaman1985 Biochem Molecular Structure of The b3 Adrenergic Receptorjames mellaleievBelum ada peringkat
- Inhibition of Peptidoglycan Biosynthesis in Gram-Positive Bacteria by LY146032Dokumen7 halamanInhibition of Peptidoglycan Biosynthesis in Gram-Positive Bacteria by LY146032SachithBelum ada peringkat
- Novel Phthalocyanines Containing SubstitDokumen10 halamanNovel Phthalocyanines Containing SubstitPopusoi AnaBelum ada peringkat
- Oxygen Free Radical Modified Dna: Implications in The Etiopathogenesis of Systemic Lupus ErythematosusDokumen8 halamanOxygen Free Radical Modified Dna: Implications in The Etiopathogenesis of Systemic Lupus ErythematosusfilianieBelum ada peringkat
- Gel Electrophoresis of ProteinsDari EverandGel Electrophoresis of ProteinsMichael J DunnBelum ada peringkat
- Giaouris Et Al POSTER CBL2007 (Rennes 13-15 Nov 2007)Dokumen1 halamanGiaouris Et Al POSTER CBL2007 (Rennes 13-15 Nov 2007)Stathis GiaourisBelum ada peringkat
- Deshmukh 2000Dokumen24 halamanDeshmukh 2000Tecno QB7Belum ada peringkat
- An 21093 LC DNAPac PA200 RS Oligonucleotides AN21093 enDokumen7 halamanAn 21093 LC DNAPac PA200 RS Oligonucleotides AN21093 enRajat DasBelum ada peringkat
- Synthesis and Activity of Metal Complexes from N-(2-Nitro) Benzylidine-3 AminocoumarinDokumen4 halamanSynthesis and Activity of Metal Complexes from N-(2-Nitro) Benzylidine-3 AminocoumarinAnantha LakshmiBelum ada peringkat
- 2016 HarmalolDokumen11 halaman2016 Harmaloltaoufik akabliBelum ada peringkat
- Hammud2008 PDFDokumen13 halamanHammud2008 PDFgringoBelum ada peringkat
- G.S. Suresh Kumar, A. Antony Muthu Prabhu, P.G. Seethalaksmi, N. Bhuvanesh, S. KumaresanDokumen8 halamanG.S. Suresh Kumar, A. Antony Muthu Prabhu, P.G. Seethalaksmi, N. Bhuvanesh, S. KumaresanAfdhonil Qomarul PBelum ada peringkat
- GKL 430Dokumen8 halamanGKL 430Anindya RoyBelum ada peringkat
- TEMED Catalyzes Polyacrylamide Gel FormationDokumen2 halamanTEMED Catalyzes Polyacrylamide Gel FormationMadhuri PatelBelum ada peringkat
- POLGDokumen11 halamanPOLGİzem DevecioğluBelum ada peringkat
- Advanced Synthesis of BiomoleculesDokumen90 halamanAdvanced Synthesis of BiomoleculesDiomedes DBelum ada peringkat
- List of References: Fractogel EMD Tentacle-PhasesDokumen54 halamanList of References: Fractogel EMD Tentacle-PhasesTuyền KimBelum ada peringkat
- Electrochemical Transduction of DNA Hybridization at Modified Electrodes by Using An Electroactive Pyridoacridone IntercalatorDokumen10 halamanElectrochemical Transduction of DNA Hybridization at Modified Electrodes by Using An Electroactive Pyridoacridone IntercalatorferBelum ada peringkat
- Application of Sodium Aluminate As A Heterogeneous Base Catalyst For Biodiesel Production From Soybean OilDokumen4 halamanApplication of Sodium Aluminate As A Heterogeneous Base Catalyst For Biodiesel Production From Soybean Oillakhya07Belum ada peringkat
- بحث رقم 50Dokumen23 halamanبحث رقم 50ahmed eldesokyBelum ada peringkat
- V. Sanz-Nebot Et Al. J. Chromatogr. B 798 2003Dokumen7 halamanV. Sanz-Nebot Et Al. J. Chromatogr. B 798 2003bookmoonBelum ada peringkat
- Iridium-Catalyzed Asymmetric Ring Opening of Azabicyclic Alkenes by AminesDokumen9 halamanIridium-Catalyzed Asymmetric Ring Opening of Azabicyclic Alkenes by AminesDiogomussumBelum ada peringkat
- Heterogeneous Asymmetric Diels-Alder Reactions Using A Copper-Chiral Bis (Oxazoline) Complex Immobilized On Mesoporous SilicaDokumen5 halamanHeterogeneous Asymmetric Diels-Alder Reactions Using A Copper-Chiral Bis (Oxazoline) Complex Immobilized On Mesoporous SilicaJC Jane BarnesBelum ada peringkat
- 1 s2.0 S0162013420301938 MainDokumen13 halaman1 s2.0 S0162013420301938 Mainstanleytiu63Belum ada peringkat
- Assessment of Heavy Metal Bioavailability Using Escherichia Coli Zntap::Lux and Copap::Lux-Based BiosensorsDokumen5 halamanAssessment of Heavy Metal Bioavailability Using Escherichia Coli Zntap::Lux and Copap::Lux-Based BiosensorsShahida ZimiBelum ada peringkat
- 2a-Basic of Genetic Engineering PDFDokumen42 halaman2a-Basic of Genetic Engineering PDFWomen 68Belum ada peringkat
- Synthesis of Novel Quaternary Chitosan Derivatives ViaDokumen4 halamanSynthesis of Novel Quaternary Chitosan Derivatives ViaAxel MéndezBelum ada peringkat
- 2005 OL OlefinesDokumen3 halaman2005 OL OlefinesCarmen SimalBelum ada peringkat
- 2005 Synthesis of DinitrochalconesDokumen4 halaman2005 Synthesis of DinitrochalconesJESUS DAVID BOLA‹O JIMENEZBelum ada peringkat
- 12 Ane Metal Ion ComplexesDokumen9 halaman12 Ane Metal Ion ComplexesSabrina MilanoBelum ada peringkat
- Ahmed 2009Dokumen7 halamanAhmed 2009shaksganeshanBelum ada peringkat
- J. Agric. Food Chem. 2012, 60, 7204 7210Dokumen7 halamanJ. Agric. Food Chem. 2012, 60, 7204 7210Duong Pham QuangBelum ada peringkat
- 17Dokumen9 halaman17'Licenza AdagioBelum ada peringkat
- C7ef PDFDokumen3 halamanC7ef PDFVishak VsBelum ada peringkat
- Tween Influence DNA HybridizationDokumen7 halamanTween Influence DNA Hybridizationsimone.bassini1996Belum ada peringkat
- Fluoresscent DNA NanotagsDokumen12 halamanFluoresscent DNA NanotagsreoloxBelum ada peringkat
- Carolyn Bertozzi's Pioneering Work in Chemical GlycobiologyDokumen40 halamanCarolyn Bertozzi's Pioneering Work in Chemical GlycobiologyMark PainterBelum ada peringkat
- Lu 2017Dokumen6 halamanLu 2017MikeBelum ada peringkat
- 2009 JOC AziridinesDokumen8 halaman2009 JOC AziridinesCarmen SimalBelum ada peringkat
- Dye-Ligand Affinity SystemsDokumen26 halamanDye-Ligand Affinity SystemsnekojcoBelum ada peringkat
- (99mTc) HYNIC-RGD for Imaging Integrin Αvβ3 ExpressionDokumen8 halaman(99mTc) HYNIC-RGD for Imaging Integrin Αvβ3 ExpressionacbgdvBelum ada peringkat
- Melting Behavior of Pseudoknots Containing Adjacent GC and AT Rich DomainsDokumen7 halamanMelting Behavior of Pseudoknots Containing Adjacent GC and AT Rich DomainsMiftahul KautsariBelum ada peringkat
- Ic401607underscoring The Influence of Inorganic Chemistry On Nuclear Imaging With RadiometalszDokumen20 halamanIc401607underscoring The Influence of Inorganic Chemistry On Nuclear Imaging With RadiometalszkawtherahmedBelum ada peringkat
- Electronic Detection of Nucleic Acids: A Versatile Platform For Molecular DiagnosticsDokumen16 halamanElectronic Detection of Nucleic Acids: A Versatile Platform For Molecular DiagnosticsLinggonilus MasturandaBelum ada peringkat
- Organotin Compound Derived From 3 Hydroxy 2 Formylpyridine Semicarbazone Synthesis Crystal Structure and Antiproliferative Activity PDFDokumen8 halamanOrganotin Compound Derived From 3 Hydroxy 2 Formylpyridine Semicarbazone Synthesis Crystal Structure and Antiproliferative Activity PDFIT InventoryBelum ada peringkat
- DNA SequencingDokumen23 halamanDNA Sequencingrr48843100% (1)
- A Stereoselective Approach To Nucleosides andDokumen4 halamanA Stereoselective Approach To Nucleosides andcarloscampanherBelum ada peringkat
- MTT - Sigma AldrichDokumen2 halamanMTT - Sigma AldrichFellicia RachmadianaBelum ada peringkat
- TBC InternasionalDokumen9 halamanTBC InternasionalDebbyZulfitrie CahyuniBelum ada peringkat
- 10 1002@aoc 5423Dokumen17 halaman10 1002@aoc 5423Roman RusnacBelum ada peringkat
- 2018 ArriortuaDokumen10 halaman2018 Arriortuajesus ibarraBelum ada peringkat
- KLOMPOKDokumen8 halamanKLOMPOKRikardoBelum ada peringkat
- Zero-Valent Metals Accelerate The Neopentylglycolborylation of Aryl Halides Catalyzed by Nicl - Based Mixed-Ligand SystemsDokumen7 halamanZero-Valent Metals Accelerate The Neopentylglycolborylation of Aryl Halides Catalyzed by Nicl - Based Mixed-Ligand SystemsDiogomussumBelum ada peringkat
- Luciferase PCR Template For IVTDokumen1 halamanLuciferase PCR Template For IVTAlleleBiotechBelum ada peringkat
- Neural Induction FactorsDokumen2 halamanNeural Induction FactorsAlleleBiotechBelum ada peringkat
- M13KO7 Helper Phage for phage displayDokumen1 halamanM13KO7 Helper Phage for phage displayAlleleBiotechBelum ada peringkat
- High Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionDokumen1 halamanHigh Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionAlleleBiotechBelum ada peringkat
- Modified UTPDokumen1 halamanModified UTPAlleleBiotechBelum ada peringkat
- ARCADokumen1 halamanARCAAlleleBiotechBelum ada peringkat
- Cre Recombinase IVT PCR TemplateDokumen1 halamanCre Recombinase IVT PCR TemplateAlleleBiotechBelum ada peringkat
- Modified CTPDokumen1 halamanModified CTPAlleleBiotechBelum ada peringkat
- Omni-Tube PCRDokumen3 halamanOmni-Tube PCRAlleleBiotechBelum ada peringkat
- Anti-RFP (5F8) Monoclonal AntibodyDokumen1 halamanAnti-RFP (5F8) Monoclonal AntibodyAlleleBiotechBelum ada peringkat
- RNA Purification SystemDokumen3 halamanRNA Purification SystemAlleleBiotechBelum ada peringkat
- Anti-RFP (3F5) Monoclonal AntibodyDokumen1 halamanAnti-RFP (3F5) Monoclonal AntibodyAlleleBiotechBelum ada peringkat
- PNCS dLanYFPDokumen1 halamanPNCS dLanYFPAlleleBiotechBelum ada peringkat
- High Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionDokumen1 halamanHigh Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionAlleleBiotechBelum ada peringkat
- Custom Sub Cloning ServiceDokumen1 halamanCustom Sub Cloning ServiceAlleleBiotechBelum ada peringkat
- Sapphire Insect Transfection ReagentDokumen1 halamanSapphire Insect Transfection ReagentAlleleBiotechBelum ada peringkat
- pmTFP1 ClathrinDokumen4 halamanpmTFP1 ClathrinAlleleBiotechBelum ada peringkat
- ChromoTek RFP BoosterDokumen1 halamanChromoTek RFP BoosterAlleleBiotechBelum ada peringkat
- Surface Bind RNA Purification SystemDokumen3 halamanSurface Bind RNA Purification SystemAlleleBiotechBelum ada peringkat
- T7 RNA PolymeraseDokumen1 halamanT7 RNA PolymeraseAlleleBiotechBelum ada peringkat
- High Titer Luc Lentiviral ParticlesDokumen1 halamanHigh Titer Luc Lentiviral ParticlesAlleleBiotechBelum ada peringkat
- Luciferase PCR Template For IVTDokumen1 halamanLuciferase PCR Template For IVTAlleleBiotechBelum ada peringkat
- iPS Induction IVT TemplatesDokumen2 halamaniPS Induction IVT TemplatesAlleleBiotechBelum ada peringkat
- RNA Purification SystemDokumen3 halamanRNA Purification SystemAlleleBiotechBelum ada peringkat
- Mwasabi PCR Template For IVTDokumen1 halamanMwasabi PCR Template For IVTAlleleBiotechBelum ada peringkat
- Allele AgaroseDokumen1 halamanAllele AgaroseAlleleBiotechBelum ada peringkat
- AvantGene Transfection KitDokumen2 halamanAvantGene Transfection KitAlleleBiotechBelum ada peringkat
- Rex1-Oct3/4-Nanog iPS Methylation Detection PrimersDokumen1 halamanRex1-Oct3/4-Nanog iPS Methylation Detection PrimersAlleleBiotechBelum ada peringkat
- ChromoTek GFP-multiTrapDokumen3 halamanChromoTek GFP-multiTrapAlleleBiotechBelum ada peringkat
- mClavGR2 Fusion VectorsDokumen3 halamanmClavGR2 Fusion VectorsAlleleBiotechBelum ada peringkat
- Biology B: Pearson Edexcel Level 3 GCEDokumen184 halamanBiology B: Pearson Edexcel Level 3 GCEaliceBelum ada peringkat
- Cell Biology: A Summary and ComparisonDokumen20 halamanCell Biology: A Summary and ComparisonMamandaBelum ada peringkat
- Protein Chemistry - Multiple Choice Questions - Set-2 Our Biochemistry - Namrata ChhabraDokumen1 halamanProtein Chemistry - Multiple Choice Questions - Set-2 Our Biochemistry - Namrata ChhabraUkikggnBelum ada peringkat
- Cell City Analogy 06Dokumen2 halamanCell City Analogy 06alek22Belum ada peringkat
- MitochondrialDokumen13 halamanMitochondrialAndrea CamachoBelum ada peringkat
- Keratinisation and CornificationDokumen38 halamanKeratinisation and Cornificationlizamjen100% (3)
- Cur 0013 0169Dokumen10 halamanCur 0013 0169Roberto SoehartonoBelum ada peringkat
- Gene TherapyDokumen5 halamanGene TherapyJamesel VillaruzBelum ada peringkat
- 9.4. CellDokumen88 halaman9.4. CellTUĞRUL ERGÜLBelum ada peringkat
- The Fundamental Unit of Life: One Mark QuestionsDokumen12 halamanThe Fundamental Unit of Life: One Mark QuestionsSrividhya ManikandanBelum ada peringkat
- Botânica de AristolochiaDokumen13 halamanBotânica de AristolochiaNatã DalarmiBelum ada peringkat
- Roche Primer DimerDokumen4 halamanRoche Primer Dimeru77Belum ada peringkat
- Genetics, Lecture 5, Trascription (Slides)Dokumen63 halamanGenetics, Lecture 5, Trascription (Slides)Ali Al-QudsiBelum ada peringkat
- Aneuploidy-Induced Delaminating Cells Drive Tumorigenesis in Drosophila EpitheliaDokumen6 halamanAneuploidy-Induced Delaminating Cells Drive Tumorigenesis in Drosophila EpitheliaPierre RICHARD-PARPAILLONBelum ada peringkat
- Chapter 8: Photosynthesis: For Questions 1-6, Complete Each Statement by Writing The Correct Word or WordsDokumen4 halamanChapter 8: Photosynthesis: For Questions 1-6, Complete Each Statement by Writing The Correct Word or WordsKrish KalraBelum ada peringkat
- Chemical Carcinogenesis I and Ii: Michael Lea 2016Dokumen31 halamanChemical Carcinogenesis I and Ii: Michael Lea 2016aasiyaBelum ada peringkat
- Viral Genome Book PDFDokumen636 halamanViral Genome Book PDFBob100% (1)
- Analysis of Pierce Amino Acid Standard H Using Vanquish FlexDokumen1 halamanAnalysis of Pierce Amino Acid Standard H Using Vanquish FlexAlexander Nieto VelaBelum ada peringkat
- Bacterial Transformation Lab (6a)Dokumen7 halamanBacterial Transformation Lab (6a)Chris PriceBelum ada peringkat
- Lecture 12' - Structural Transitions in Nucleic Acids IIDokumen39 halamanLecture 12' - Structural Transitions in Nucleic Acids IIcurlicueBelum ada peringkat
- Biology - Canizales - 1(A) Lab: Albinism QuizDokumen1 halamanBiology - Canizales - 1(A) Lab: Albinism QuizArijan SchiffererBelum ada peringkat
- Functions of the Plasma Membrane and Fluid Mosaic ModelDokumen19 halamanFunctions of the Plasma Membrane and Fluid Mosaic ModelKrystin DiamosBelum ada peringkat
- 2219 Final ReportDokumen38 halaman2219 Final ReportRicardo ParralBelum ada peringkat
- Activation of mTOR Signalling in Young and Old Human Skeletal Muscle in Response To Combined Resistance Exercise and Whey Protein IngestionDokumen10 halamanActivation of mTOR Signalling in Young and Old Human Skeletal Muscle in Response To Combined Resistance Exercise and Whey Protein IngestionCarlos RodriguezBelum ada peringkat
- Informasi Harga ONT 2022Dokumen5 halamanInformasi Harga ONT 2022RizkiBelum ada peringkat
- Practice Exam 3A BMB 401Dokumen6 halamanPractice Exam 3A BMB 401rajBelum ada peringkat
- Restriction Enzyme Mapping Virtual LabDokumen6 halamanRestriction Enzyme Mapping Virtual Labapi-522847737Belum ada peringkat
- All-Natural 5-Meo-Dmt Sigma Receptor 1 Agonist and Its Therapeutic Impact in Mental and Neurodegenerative Diseases Through Mitochondrial ActivationDokumen22 halamanAll-Natural 5-Meo-Dmt Sigma Receptor 1 Agonist and Its Therapeutic Impact in Mental and Neurodegenerative Diseases Through Mitochondrial ActivationLex UBelum ada peringkat
- Questions - Enzymes - Answer KeyDokumen7 halamanQuestions - Enzymes - Answer KeyBUG50% (2)
- TEST BANK - Chapter 3 - Amino Acids, Peptides, and ProteinsDokumen31 halamanTEST BANK - Chapter 3 - Amino Acids, Peptides, and ProteinsAndreaBelum ada peringkat