Targeted Therapies CCR2008
Diunggah oleh
Marie ChaisanaDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Targeted Therapies CCR2008
Diunggah oleh
Marie ChaisanaHak Cipta:
Format Tersedia
Perspective
Targeted Therapies to Improve Tumor Immunotherapy
Jonathan Begley1,2 and Antoni Ribas2,3,4
Abstract Durable tumor regression and potential cures of metastatic solid cancers can be achieved by a
variety of cellular immunotherapy strategies, including cytokine therapy, dendritic cell ^ based
vaccines, and immune-activating antibodies, when used in so-called immune-sensitive cancers
such as melanoma and renal cell carcinoma. However, these immunotherapy strategies have very
low tumor response rates, usually in the order of 5% to 10% of treated patients.We propose that
the antitumor activity of adequately stimulated tumor antigen ^ specific T cells is limited by local
factors within the tumor milieu and that pharmacologic modulation of this milieu may overcome
tumor resistance to immunotherapy. By understanding the mechanisms of cancer cell immune
escape, it may be possible to design rational combinatorial approaches of novel therapies able
to target immunosuppressive or antiapoptotic molecules in an attempt to reverse resistance to
immune system control. We term this mode of treatment ‘‘immunosensitization.’’ Ideal candidates
for immunosensitizing drugs would be targeted drugs that block key oncogenic mechanisms
in cancer cells resulting in a proapoptotic cancer cell milieu and at the same time do not nega-
tively interfere with critical lymphocyte functions.
One of the hallmarks of cancer responses to cellular immu- fight immune resistance and allow the durable responses seen
notherapy is that they are extremely long lived, frequently with immunotherapy to take hold.
measured in years. However, objective responses (as opposed to
the more common claim of mixed responses or disease
stabilization with immunotherapy) are rare, usually noted in Limiting Steps for Effective Tumor
a minority of patients, in the range of 5% to 10% (1, 2). The Immunotherapy
improved understanding of the oncogenic events in cancer
The multiple advances in understanding the immunobiology
and the development of multiple new highly targeted drugs of T-cell responses to cancer have not translated into wide-
that block or neutralize oncogenic factors open the door for spread clinical utility (1). Cytokine-based therapies, dendritic cell
potential novel combinatorial approaches with immunother- (DC)-based vaccines, and treatment with immune-activating
apy. In this article, we postulate that targeted agents against antibodies, particularly with blocking antibodies to the CTL-
key immunosuppressive, oncogenic, or antiapoptotic factors associated protein 4 (CTLA4), have a ceiling of tumor responses
in cancer cells can sensitize cancers to immunotherapy. We in a subset of so-called immune-sensitive tumors (mainly
restrict the definition of tumor immunotherapy to mean to melanoma and renal cell carcinoma) in the range of 5% to 10%
activate cellular cytotoxic antitumor immune responses, mostly of treated patients (1, 2). However, enthusiasm for tumor
mediated by CD8+ CTLs and natural killer (NK) cells. This is immunotherapy is maintained by the realization that most
opposed to therapy mediated by antibodies such as rituximab, tumor responses are durable, leading to rare chances of cure in
trastuzumab, or bevacizumab, which target surface or secreted patients with widely metastatic solid tumors. That is the basis
proteins in cancer cells but are not designed to induce adaptive for the Food and Drug Administration licensing of the use of
immune responses to the cancer. We suggest that optimal high-dose interleukin (IL)-2 in metastatic melanoma and renal
combinations of these drugs and cellular immunotherapy cell carcinoma (3), and consistent with the findings emerging
will result in improved antitumor responses as targeted drugs from ongoing clinical studies developing DC-based vaccines
(2, 4) and anti-CTLA4 antibodies (5, 6). Therefore, although we
have the proof of concept about the effectiveness of tumor
Authors’ Affiliations: 1Monash University, Victoria, Melbourne, Australia; and
Departments of 2Surgery and 3Medicine, University of California at Los Angeles;
immunotherapy, major improvements are needed in many key
and 4Jonsson Comprehensive Cancer Center, Los Angeles, California aspects of this type of therapy.
Received 11/2/07; revised 1/7/08; accepted 1/23/08. There are many factors that influence tumor response to
Grant support: Harry J. Lloyd Charitable Trust and Melanoma Research immunotherapy. Neoplastic cells survive and proliferate in an
Foundation.
often hostile environment. Under constant selection pres-
The costs of publication of this article were defrayed in part by the payment of page
charges. This article must therefore be hereby marked advertisement in accordance sure, cells with a survival advantage replicate, thus ensuring
with 18 U.S.C. Section 1734 solely to indicate this fact. the tumor ‘‘evolves’’ to suit its environment. Tumor cells that
Requests for reprints: Antoni Ribas, Division of Hematology-Oncology, develop mechanisms to evade the immune system allow the
University of California at Los Angeles Medical Center, 11-934 Factor Building, cancer to become immunoresistant in a process known as
10833 Le Conte Avenue, Los Angeles, CA 90095-1782. Phone: 310-206-3928;
Fax: 310-206-0914; E-mail: aribas@ mednet.ucla.edu.
cancer immunoediting (7). Within this context, the ability
F 2008 American Association for Cancer Research. of immune system cells to attack cancers is limited. Figure 1
doi:10.1158/1078-0432.CCR-07-4804 depicts a hypothetical model of tumor immunotherapy
www.aacrjournals.org 4385 Clin Cancer Res 2008;14(14) July 15, 2008
Perspective
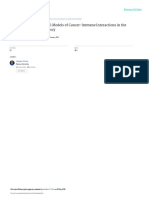
Fig. 1. Modelof CTL response to melanoma.
There are several steps required for the
induction of an effective antitumor immune
response: (1) antigen-presenting cells need
to activate CD8+ tumor-specific effector cells
overcoming the limitations of targeting
tumor self-antigens; (2) CD8+ cells need to
overcome homeostatic mechanisms that
limit immune activation, and then they need
to expand and traffic to tumor targets; (3)
CD8+ cells have to recognize antigen
presented by tumor cells and (4) overcome
tumor-induced local negative immune
regulators; and (5) the tumor cells need to be
sensitive to the lytic effects of the immune
effector cells, delivered either through
perforin/granzyme or by death receptor
ligation (Fas,TRAIL, andTNF). GzB,
granzyme B;Treg,Tregulatory cell;VEGF,
vascular endothelial growth factor; PgE2,
prostaglandin E2;TGF-h, transforming
growth factor-h;TAP, transporter associated
with antigen processing; PD1, programmed
death1.
highlighting the key steps in generating an effective antitumor critically requires the delivery of costimulatory signals provided
immune response. At each step, the immune system or the by B7 molecules (CD80 and CD86) recognized by their
tumor may limit or prevent the effective elimination of cancer positive receptor CD28 on T cells. The coinhibitory molecule
cells by T cells. Obviously, this is a simplified model given the CLTA4 (CD152) has a pivotal role in dampening immune
complexity of the immune response and the multiple cell responses to self-antigens by competing with CD28 (12).
subsets involved. However, we focus on the key interactions Blocking antibodies to CTLA4 are in clinical development and
leading to an effective adaptive immune response to cancer to have shown durable immune-mediated response rates in the
help describe what we believe are the key limitations to current order 5% to 20% in patients with metastatic melanoma (5, 6,
immunotherapy. 13, 14). Several other costimulatory and coinhibitory targets are
T-cell activation by antigen-presenting cells. The first step in amenable to intervention, and specific antibodies against
generating an effective antitumor immune response leads to the CD40, CD80, CD134 (OX40), CD137 (41BB), programmed
licensing of CD8+ CTLs, which, through the T-cell receptor, death 1, and others are being tested (10). Therefore, several
specifically recognize their cognate antigen presented by tumor off-the-shelf potent immunostimulating agents are entering
cells through MHC class I molecules. Most tumor antigens are clinical testing for cancer, making it feasible the future study of
self-antigens (i.e., antigen normally expressed on healthy cells combinatorial approaches.
and shared by cancer cells). T cells recognizing these self- T-cell expansion and tumor targeting. After CD8+ CTLs are
antigens would cause autoimmunity; self-reactive T cells are activated against cells expressing tumor antigens, they need to
subjected to negative selection in the thymus and are only overcome homeostatic mechanisms that limit immune activa-
detectable in very low levels in the periphery. In addition, tion and expansion. They then traffic to tumors. The immune
tumor antigens are frequently down-regulated by cancer cells system has multiple breaks limiting this activation process,
and do not themselves lead to activation of tumor antigen – including the expression of CTLA4 by activated T cells leading
specific CTL (8). For these antigens to be recognized, they need to interference in establishing lasting interactions between
to be presented by DC to CD4+ T helper cells or cross-presented activated T cells and target cells expressing their cognate
(a process in which exogenous antigen is presented in MHC antigen (15).
class I) by DC to CD8+ CTL (9) in an interaction modulated by Recognition of cancer cells. Activated tumor antigen – specific
costimulatory and coinhibitory molecules (10). The develop- CD8+ cells that have traveled to the tumor have to then recog-
ment of methods to generate DC ex vivo allowed their testing as nize their cognate antigen presented by tumor cells to speci-
a powerful means to activate the immune system to cancer self- fically deliver their cytotoxic signals. The tumor has developed
antigens (11). The clinical results to date are suboptimal (1) elaborated mechanisms to escape the immune system. Low-
and highlight the need to address other variables involved level expression of antigen, MHC molecules (HLA in humans),
in immune-mediated tumor regression other than the use of h2-microglobulin required for MHC assembly, or transporter
powerful vaccines. associated with antigen processing molecules required to
Costimulatory and coinhibitory molecules. Antigen presenta- provide peptide epitopes for MHC stability on the cell surface,
tion by MHC recognized by T-cell receptors on T lymphocytes is may result in CD8+ CTL that cannot find their cognate antigen
not enough to activate an adaptive immune response; it on cancer cells (16).
Clin Cancer Res 2008;14(14) July 15, 2008 4386 www.aacrjournals.org
Immunosensitization with Targeted Therapies
Immunosuppressive tumor milieu. Local immunosuppressive with caspase-8 for binding to the death-inducing signaling
factors within the tumor milieu include secreted molecules, complex. Expression of FLIP in cancer cells renders them insen-
such as transforming growth factor-h, IL-10, prostaglandin sitive to Fas ligand or TRAIL. Bax or Bak expression is signifi-
E2, and vascular endothelial growth factor (VEGF). These cantly reduced in many cancers and confers worse prognosis
factors are produced either by the tumor cells themselves or by (26). Low expression of Apaf-1 (a proapoptotic molecule),
surrounding host stromal cells on tumor cell signaling. The probably through methylation, is associated with chemore-
suppressive tumor environment is further maintained by sistance (27). Equally, down-regulation of the death signal-
immunosuppressive cells, such as T regulatory cells, and ing molecule Fas-associated protein with death domain and
indolamine 2,3-dioxygenase (IDO)-producing plasmacytoid caspase-8 has been shown in several cancers (28, 29). As
DC (17, 18). These are professional immune suppressor cells expected, up-regulation of inhibitors of apoptosis (antiapop-
that are frequently found inside tumors and provide means for totic molecules that inhibit the effector caspases) is overex-
tumor escape from activated lymphocytes. pressed in tumor cells (30, 31).
Escape from immune effector cell killing of target cells. Im-
mune effector cells (either CD8+ CTL or NK cells) kill their Therapeutics with Immunopotentiating Profile
targets by four defined mechanisms: the release of perforin and
granzymes (see ref. 19 for review) and the engagement of the With an understanding of the potential role of the cellular
Fas, tumor necrosis factor (TNF)-related apoptosis-inducing immune system in detecting and killing cancer cells, and
ligand (TRAIL), and TNF death receptors (see ref. 20 for insight into how cancer cells avoid death from the immune
review). A simplified schematic of this process is shown system, pharmacologic interventions may be designed to
in Fig. 2. On entry into target cells facilitated by perforin, sensitize cancer cells to immune attack. Table 1 provides the
granzyme B directly cleaves Bid (a proapoptotic Bcl-2 family features that we feel would make up an ideal immunosensitiz-
member) resulting in activation of caspase-3 and other ing drug. It should potentiate the immune effector cell
downstream effector caspases, leading to apoptotic cell death. recognition of cancer cells and shift the balance in the cancer
Engagement of the surface Fas, TRAIL, or TNF death receptors cell toward a proapoptotic milieu. Of particular importance,
by their secreted or cell surface – anchored ligands presented on potential immunosensitizing drugs must not be cytotoxic
immune effector cells leads to the activation of caspase-8 within against nor inhibit critical functions of immune cells.
the target cell and activation of the extrinsic pathway of Decreasing tumor-induced immune suppression. Secreted
apoptosis. Caspase-8 also activates the intrinsic (also called proteins released by tumors that lead to immune escape can
‘‘mitochondrial’’) apoptosis pathway (see ref. 21 for review), be targets to monoclonal antibody blockade. For example, the
which depends heavily on the balance between proapoptotic anti – VEGF antibody bevacizumab, in addition to its effect of
and antiapoptotic molecules, particularly of the Bcl-2 family. tumor vasculature, may decrease VEGF – induced inhibition
Intracellular effectors of cytotoxicity and apoptotic cell of DC and T-cell function (32). Monoclonal antibodies to
death. The fate of a cell reflects the dynamic interplay between transforming growth factor-h and IL-10 are also potential
apoptosis and survival; the Bcl-2 family is critical in maintain- means to reverse immune escape induced by tumor-released
ing this balance. The family is classified into three groups: the molecules. In addition, anti-inflammatory drugs such as aspirin
‘‘antiapoptotic,’’ ‘‘proapoptotic,’’ and ‘‘BH3-only’’ proteins (see and other nonsteroidal anti-inflammatory drugs, including the
ref. 22 for further review). The antiapoptotic Bcl-2 family specific cyclooxygenase-2 inhibitor celecoxib, could be means to
members include Bcl-2, Bcl-XL, Bcl-w, Bcl-2A1, Mcl1, and Boo, inhibit cyclooxygenase-2 that leads to prostaglandin E2 produc-
which promote cell survival by antagonizing apoptotic signals. tion (33).
Overexpression of these molecules protects cells from apoptosis Depletion of immunosuppressive cells. T regulatory cells,
and mediates cell survival (23). The proapoptotic Bcl-2 family myeloid suppressor cells, and IDO-positive plasmacytoid DC
members include Bax, Bak, Box, and Bcl-XS. Overexpression of have emerged as major immunosuppressive cells reported to be
these molecules has been shown to promote apoptosis (23). implicated in escape of tumors from immune control (34, 35).
These molecules are directly responsible for initiating apoptosis Depletion of CD4/CD25/FoxP3-positive natural T regulatory
in the mitochondrial pathway and are considered important cells can be achieved in mice using anti-CD25 antibodies,
sensors of cellular stress (such as DNA damage). The BH3-only which leads to improvement in responses to immunostimulat-
subfamily is proapoptotic but is classified separately due to its ing approaches such as CTLA4 blockade (36). There is much
mechanism of action. It includes Bad, Bik, Bid, Hrk, Bim, Noxa, interest in developing means to deplete human T regulatory
Puma, and Bmf. The primary role of these molecules is the cells, although no compelling approach has yet been devel-
activation of the proapoptotic family members. oped. The available antibodies to human CD25 approved by
Cancer resistance to apoptosis. Given the critical role of the Food and Drug Administration are immunosuppressive as
proapoptotic and antiapoptotic proteins, it is not surprising opposed to immunostimulating, and there are mixed reports
that altered expression of these proteins in cancerous cells often on the ability of a CD25-targeted IL-2-diphtheria toxin fusion
allows them to resist apoptosis. Fas and TRAIL receptors are protein (denileukin diftitox) to deplete T regulatory cell func-
down-regulated or undetectable in many cancers, obviously tion (37). The function of the immunosuppressive enzyme IDO
leading to resistance to death receptor – induced apoptosis in can be inhibited by 1-methyl-tryptophan (17), and clinical
most cases (24). The nuclear factor-nB is a transcription factor trials testing its effects in patients with cancer are planned. This
with constitutive expression in many cancers (25) and is reagent would have the potential of synergizing with immu-
responsible for the transcription of many antiapoptotic notherapy by inhibiting immune suppression by IDO.
genes, including the FLICE inhibitory protein (FLIP). FLIP is Increase activating ligands for immune effector cells. Drugs
expressed at high levels in many cancers, where it competes that mediate epigenetic mechanisms, such as the demethylating
www.aacrjournals.org 4387 Clin Cancer Res 2008;14(14) July 15, 2008
Perspective
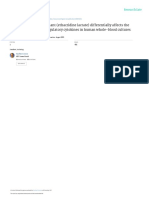
Fig. 2. Apoptotic pathways within the normal cell.
Cytotoxic immune cells can induce target cell death by
engaging death receptors or releasing perforin and
granzymes (Grn). This schematic shows a simplification
of the signaling activated by this process. DISC, death-
inducing signaling complex; FADD, Fas-associated
protein with death domain; IAP, inhibitor of apoptosis.
agents and the histone deacetylase inhibitors, have multiple (39). This would suggest that ubiquitin ligase inhibitors and
effects on gene transcription. They tend to promote a pro- proteasome inhibitors may decrease the pool of peptides able
apoptotic phenotype, can up-regulate MHC and tumor antigen to support assembly of surface MHC class I molecules.
expression, induce ‘‘stress’’ molecules recognizable by NK- Decreased expression of surface MHC class I allows cancer
activating receptors such as NKG2D, and increase expression of cells to escape from CD8+ T-cell control, but at the same time,
surface death receptors (38). The ability of this classes of novel they become more sensitive to NK cell recognition and killing.
agents to increase the surface expression of antigen-presenting NK cells are effector cells of the innate immune system, which
MHC molecules (HLA molecules in humans) and at the same sense the loss of surface MHC molecules (frequent after viral
time increase expression of tumor antigens, such as the MAGE infections and in cancer cells) or the presence of foreign MHC
family of cancer-testis antigens, may allow these agents to over- molecules (as occurs after organ transplants), which trigger
come a major mechanism of tumor escape to cellular these potent cytotoxic cells. This possibility has been tested in
immunotherapy (16). an experimental model of melanoma, suggesting that the
Decreased inhibitory ligands for immune effector cells. Pep- proteasome inhibitor bortezomib increased TNF-a – mediated
tides derived from proteasomal degradation are required for killing by immune cells (40).
assembly of MHC molecules in the endoplasmic reticulum Direct modulation of apoptotic pathways. Death receptors
are on the outer surface of cells and therefore accessible to
monoclonal antibodies. TRAIL receptors seem to be preferen-
Table 1. Ideal attributes of targeted tially expressed by cancer cells (and activated lymphocytes),
immunosensitizing drugs whereas Fas and TNF-a receptors are expressed more
promiscuously. A soluble TRAIL ligand construct and TRAIL
Effects on tumor cells receptor-activating antibodies are in clinical development for
Specifically target a key oncogenic pathway the treatment of cancer. These approaches may be viewed as
Increase death receptor expression
bypassing the need for immune effector cells but would be
Increase proapoptotic molecules
Inhibit antiapoptotic molecules unlikely to lead to sustained tumor regression without chronic
Increase expression of tumor antigen or dosing. It is possible that such modulation of TRAIL receptors
NK-activating receptors may synergize with immunotherapy and other targeted therapy
Effects on immune system cells approaches. Histone deacetylase inhibitors have been shown
No cytotoxic effects on immune system cells
No interference with T-cell receptor, NK receptor,
to up-regulate the TRAIL death receptor DR5, leading to
or costimulatory signal transduction pathways sensitization of cancer cells to TRAIL-induced death (41, 42),
and therefore would be logical drugs to use in combination with
Clin Cancer Res 2008;14(14) July 15, 2008 4388 www.aacrjournals.org
Immunosensitization with Targeted Therapies
both TRAIL-engaging reagents and immune effector cells. kinase constitutively activated in PTEN-deficient cells) re-
Furthermore, the demethylating agent decitabine has been versed immune escape of glioblastoma cells (54). However,
shown to sensitize choroidal melanoma cell lines to the cyto- in addition to its key role in oncogenesis, the phosphatidy-
toxic effects of IFNs by inducing the expression of proapoptotic linositol 3-kinase-Akt-mammalian target of rapamycin signal-
and growth-inhibitory genes (43). ing pathway is key to many lymphocyte functions. If targeted
The antiapoptotic members of the Bcl-2 family are potential drugs blocking this signal transduction pathway are to be
targets for immunosensitizing drugs. Bcl-2 is up-regulated in used as immune sensitizers, they will need to be carefully
many cancers (44), and inactivating its function may result in a scheduled or will need to show a high therapeutic window to
proapoptotic cancer cell milieu facilitating the cytotoxic effect have the required effect on cancer cells without suppressing
of immune cells. Small-molecule inhibitors of Bcl-2 family lymphocyte functioning.
members (45) have obvious pharmacologic advantages over The signal transducer and activator of transcription (STAT)
Bcl-2 antisense oligonucleotides (46) and may be logical proteins transmit cytoplasmic signals from polypeptide cyto-
compounds to be used in combination with immunotherapy. kines and growth factors that have receptors with intrinsic or
Other proteins in the apoptotic pathways also represent associated tyrosine kinase activity. They have become promis-
potential targets for immunosensitizing agents. An inhibitor ing molecular targets for cancer because they are persistently
of apoptosis inhibitor sensitized cells to TRAIL (47), making it a activated in many cancers due to oncogene signaling through
reasonable drug to combine with immunotherapy. Apoptotic STATs, especially STAT-3 and STAT-5 (55). These two STAT pro-
pathways may also be targeted by altering transcription of teins have key roles in regulating cell proliferation, resistance to
regulators of apoptosis. Inhibition of nuclear factor-nB in apoptosis, and sustained angiogenesis (55). In addition, STAT-3
melanoma sensitizes cells to TRAIL-induced (but not Fas or has an important role in the immune system. Inhibiting STAT-3
TNF-a) apoptosis (48). An additional approach would be the in immune system cells increased DC, T-cell, and NK cell
use of the proteasome inhibitor bortezomib, which is known to function (56). Small-molecule inhibitors are being developed
inhibit nuclear factor-nB and down-regulate the antiapoptotic to block STAT-3 and would therefore have the potential of
molecule FLIP (49). simultaneously attacking cancer cells and increasing immune
system function (56).
Alteration of Oncogenic Pathways Leading to
Immune Sensitization Issues About the Testing of Targeted Therapy-
Immunotherapy Combinatorial Approaches
Many interventions that damage cancer cells lead to a
proapoptotic milieu. Carefully sequenced treatment with Despite the promise, there are several practical limitations to
cytotoxic drugs or radiation therapy leading to DNA damage the combined testing of new agents in preclinical or clinical
stimulates DNA stress pathways, which can lead to immune development and tumor immunotherapy approaches. Preclin-
sensitization (50, 51). In cells that have functional p53, DNA ical testing of a potentially immunosensitizing pharmacologic
damage is sensed by p53, resulting in increased transcription of agent and immunotherapy is best done in fully immunocom-
death receptors (52). Although the adverse effects of standard petent animal models. Ex vivo cell culture systems cannot fully
chemotherapy agents and radiation therapy, which are non- recapitulate a functional immune system and may provide
specific genotoxins, on immune system cells make combina- biased results, where potential adverse effects of the drug on
tions of chemotherapy and immunotherapy a rather poor immune system cells may not be detected. However, implant-
match, when adequately sequenced there may be situations able murine tumors may not be an adequate model for human
when cytotoxic therapy can be efficiently delivered to improve cancer because the oncogenic events that drive the cancers and
on the antitumor activity of immunotherapy (51, 53). the relation between the cancer cells and the tumor milieu may
Novel targeted therapies that disrupt dominant oncogenic not be the same as in spontaneously arising tumors. A notable
signals in cancer cells may result in increased sensitivity to example is the frequently used B16 murine melanoma, which
immune responses. There is a lot of interest in finding ways to does not seem to have a constitutively active mitogen-activated
combine tyrosine kinase inhibitors, such as sorafenib and protein kinase pathway, a marked difference with human
sunitinib, with immunostimulating cytokines in the treatment melanomas where mutually exclusive activating mutations on
of renal cell carcinoma. A careful assessment of the effects on the Nras of Braf are prevalent. The use of human tumor xenografts
tumor, tumor microenvironment, and the immune system will be requires implantation into severe immunodeficient mice,
required to define optimal combinations. Several clinical trials are obviously devoid of an immune system. Regeneration of a
ongoing where these multitargeted tyrosine kinase inhibitors human immune system in these mice is technically challenging,
are tested in combination with immunostimulating agents, such and current approaches may not provide a fully functional
as IL-2, IL-21, anti-CTLA4 antibodies, and poxvirus vaccines, immune response to test immune sensitization approaches.
in patients with metastatic renal cell carcinoma and melanoma. Another important limitation to the testing of the immune
Inhibition of constitutively activated signal transduction sensitization combinatorial approach is access to adequate
pathways in cancer cells also has the potential to decrease compounds. It is widely known that intellectual property issues
tumor escape from immunotherapy. For example, loss of the limit the availability of emerging new drugs, and the testing of
tumor suppressor PTEN in glioblastoma cells leads to combination regimens is frequently explicitly prohibited by the
immune escape in part by increased translation of the manufacturers of the novel agents. Because the combination is
immune suppressor surface receptor B7-H1 (CD274, also only relevant if there is a fully functioning immune system, the
known as programmed death ligand 1). Treatment of these concept frequently needs to be tested in a living animal. The
cells with an Akt inhibitor (which blocked the Akt tyrosine doses required for treatment in animal models are much higher
www.aacrjournals.org 4389 Clin Cancer Res 2008;14(14) July 15, 2008
Perspective
than the amount of agent that would be sufficient for in vitro which there is mechanistic understanding of how they
testing, so the immune sensitization combinatorial approach is potentiate each approach will be likely to be tested first. In
unlikely to be tested unless the manufacturer provides enough particular, pharmacologic means to affect tumor escape from
agent and explicit permission for combined agent testing. cellular immunotherapy would be a high priority for clinical
Alternatives such as using a published chemical structure to testing.
synthesize the agent de novo are likely to be limited by the high
costs of producing large amounts for testing in animal models.
Therefore, this approach usually requires a material transfer Conclusions
agreement with the manufacturer of the agent, with its resulting
Improvements in our understanding of the antiapoptotic
delay and limitations for combination testing. Testing in the
and immune escape mechanisms activated by oncogenic
clinic is also subjected to practical limitations. The same issues
events, and the ability to specifically alter them with targeted
related to the intellectual property of a combination described
drugs, will result in novel approaches allowing rational
for the preclinical testing apply to the clinic. Even when
combinations of drugs with immunostimulating effects. These
permission is obtained to test a new combination for which
combinations would bring together the elegance of targeted
there is strong scientific rationale, the combined testing may be
therapy for cancer with the ability of the immune system to
delayed until the safety, toxicity, and antitumor activity of each
lead to long-term regressions in some patients with metastatic
agent has been thoroughly tested independently.
cancers.
Despite all of these limitations, it is likely that in the next
several years the number of articles describing combinations of
novel targeted agents with established or new immunotherapy Disclosure of Potential Conflicts of Interest
approaches will continue to increase. This will be greatly
A. Ribas has received research funding and honoraria from Pfizer Inc.
facilitated once the novel agents have proven their benefit in
certain cancers and become standard of care therapies, as
exemplified by the ongoing clinical testing of multitargeted
Acknowledgments
tyrosine kinase inhibitors and cytokines. At some point, there
may be a need for prioritization of the combinations. It is clear We thank Glenn Begley for reviewing the manuscript and Benjamin Bonavida,
that combinations successfully tested in animal models for Hermes Garban, and James S. Economou for their valuable discussions.
References
1. Rosenberg SA,Yang JC, Restifo NP. Cancer immuno- responses in a phase I trial with the anti-cytotoxic 25. Baeuerle PA, Baltimore D. NF-nB: ten years after.
therapy: moving beyond current vaccines. Nat Med T lymphocyte-associated antigen 4 monoclonal anti- Cell 1996;87:13 ^ 20.
2004;10:909 ^ 15. body CP-675,206. JClin Oncol 2005;10:8968 ^ 77. 26. Fecker LF, Geilen CC, Tchernev G, et al. Loss of
2. Ribas A. Update on immunotherapy for melanoma. 14. Downey SG, Klapper JA, Smith FO, et al. Prog- proapoptotic Bcl-2-related multidomain proteins in
J Natl Compr Canc Netw 2006;4:687 ^ 94. nostic factors related to clinical response in patients primary melanomas is associated with poor progno-
3. Tsao H, Atkins MB, Sober AJ. Management of cuta- with metastatic melanoma treated by CTL-associat- sis. J Invest Dermatol 2006;126:1366 ^ 71.
neous melanoma. N Engl JMed 2004;351:998 ^ 1012. ed antigen-4 blockade. Clin Cancer Res 2007;13: 27. Soengas MS, Lowe SW. Apoptosis and melanoma
4. Timmerman JM, Levy R. Dendritic cell vaccines for 6681 ^ 8. chemoresistance. Oncogene 2003;22:3138 ^ 51.
cancer immunotherapy. Annu Rev Med 1999;50: 15. Schneider H, Downey J, Smith A, et al. Reversal of 28. TeitzT,Wei T,Valentine MB, et al. Caspase 8 is delet-
507 ^ 29. the TCR stop signal by CTLA-4. Science 2006;313: ed or silenced preferentially in childhood neuroblasto-
5. Korman AJ, Peggs KS, Allison JP. Checkpoint block- 1972 ^ 5. mas with amplification of MYCN. Nat Med 2000;6:
ade in cancer immunotherapy. Adv Immunol 2006;90: 16. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. 529 ^ 35.
297 ^ 339. Escape of human solid tumors from T-cell recogni- 29. Tourneur L, Mistou S, Michiels FM, et al. Loss of
6. Ribas A, Hanson DC, Noe DA, et al. Tremelimumab tion: molecular mechanisms and functional signifi- FADD protein expression results in a biased Fas-
(CP-675,206), a cytotoxic T lymphocyte associated cance. Adv Immunol 2000;74:181 ^ 273. signalingpathway and correlates with thedevelopment
antigen 4 blocking monoclonal antibody in clinical 17. Munn DH, Mellor AL. IDO and tolerance to tumors. of tumoral status in thyroid follicular cells. Oncogene
development for patients with cancer. Oncologist Trends Mol Med 2004;10:15 ^ 8. 2003;22:2795 ^ 804.
2007;12:873 ^ 83. 18. Wang HY, Lee DA, Peng G, et al. Tumor-specific hu- 30.Vucic D, Stennicke HR, Pisabarro MT, Salvesen GS,
7. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. man CD4+ regulatoryTcells and their ligands: implica- Dixit VM. ML-IAP, a novel inhibitor of apoptosis that is
Cancer immunoediting: from immunosurveillance to tions for immunotherapy. Immunity 2004;20:107 ^ 18. preferentially expressed in human melanomas. Curr
tumor escape. Nat Immunol 2002;3:991 ^ 8. 19. Trapani JA, Smyth MJ. Functional significance of Biol 2000;10:1359 ^ 66.
8. Pardoll DM. Inducing autoimmune disease to treat the perforin/granzyme cell death pathway. Nat Rev 31. Zhang XD, Zhang XY, Gray CP, NguyenT, Hersey P.
cancer. Proc Natl Acad Sci U S A 1999;96:5340 ^ 2. Immunol 2002;2:735 ^ 47. Tumor necrosis factor-related apoptosis-inducing li-
9. Carbone FR, Kurts C, Bennett SR, Miller JF, Heath 20. Dempsey PW, Doyle SE, HeJQ, Cheng G.The signal- gand-induced apoptosis of human melanoma is regu-
WR. Cross-presentation: a general mechanism for ingadaptorsandpathwaysactivatedbyTNFsuperfamily. lated by smac/DIABLO release from mitochondria.
CTL immunity and tolerance. Immunol Today 1998; Cytokine Growth Factor Rev 2003;14:193 ^ 209. Cancer Res 2001;61:7339 ^ 48.
19:368 ^ 73. 21. Kim R. Recent advances in understanding the cell 32. Gabrilovich D, Ishida T, Oyama T, et al. Vascular en-
10. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, death pathways activated by anticancer therapy. Can- dothelial growth factor inhibits the development of
Chen L. Immunostimulatory monoclonal antibodies cer 2005;103:1551 ^ 60. dendritic cells and dramatically affects the differentia-
for cancer therapy. Nat Rev Cancer 2007;7:95 ^ 106. 22. Marsden VS, Strasser A. Control of apoptosis in the tion of multiple hematopoietic lineages in vivo. Blood
11. Steinman RM, Mellman I. Immunotherapy : immune system: Bcl-2, BH3-only proteins and more. 1998;92:4150 ^ 66.
bewitched, bothered, and bewildered no more. Sci- Annu Rev Immunol 2003;21:71 ^ 105. 33. Sharma S, Stolina M,Yang SC, et al. Tumor cycloox-
ence 2004;305:197 ^ 200. 23. Strasser A. The role of BH3-only proteins in ygenase 2-dependent suppression of dendritic cell
12. Chambers CA, Kuhns MS, Egen JG, Allison JP. the immune system. Nat Rev Immunol 2005;5: function. Clin Cancer Res 2003;9:961 ^ 8.
CTL A-4-mediated inhibition in regulation of T 189 ^ 200. 34. Munn DH, Sharma MD, Hou D, et al. Expression of
cell responses: mechanisms and manipulation in 24. Zhang XD, Franco A, Myers K, et al. Relation of indoleamine 2,3-dioxygenase by plasmacytoid den-
tumor immunotherapy. Annu Rev Immunol 2001;19: TNF-related apoptosis-inducing ligand (TRAIL) re- dritic cells in tumor-draining lymph nodes. J Clin Invest
565 ^ 94. ceptor and FLICE-inhibitory protein expression to 2004;114:280 ^ 90.
13. Ribas A, Camacho LH, Lopez-Berestein G, et al. TRAIL-induced apoptosis of melanoma. Cancer Res 35. Curiel TJ, Coukos G, Zou L, et al. Specific recruit-
Antitumor activity in melanoma and anti-self 1999;59:2747 ^ 53. ment of regulatory T cells in ovarian carcinoma fosters
Clin Cancer Res 2008;14(14) July 15, 2008 4390 www.aacrjournals.org
Immunosensitization with Targeted Therapies
immune privilege and predicts reduced survival. Nat APO2-L in human malignant tumor cells. Oncogene inhibitor PS-341 sensitizes neoplastic cells to TRAIL-
Med 2004;10:942 ^ 9. 2004;23:6261 ^ 71. mediated apoptosis by reducing levels of c-FLIP.
36. Sutmuller RP, van Duivenvoorde LM, van Elsas A, 42. Insinga A, Monestiroli S, Ronzoni S, et al. Inhib- Blood 2003;102:303 ^ 10.
et al. Synergism of cytotoxicT lymphocyte-associated itors of histone deacetylases induce tumor-selective 50. Frost PJ, Butterfield LH, DissetteVB, Economou JS,
antigen 4 blockade and depletion of CD25(+) reg- apoptosis through activation of the death receptor Bonavida B. Immunosensitization of melanoma tumor
ulatory T cells in antitumor therapy reveals alternative pathway. Nat Med 2005;11:71 ^ 6. cells to non-MHC Fas-mediated killing by MART-1-
pathways for suppression of autoreactive cytotoxic 43. Gollob JA, Sciambi CJ. Decitabine up-regulates specific CTL cultures. J Immunol 2001;166:3564 ^ 73.
T lymphocyte responses. J Exp Med 2001;194: S100A2 expression and synergizes with IFN-g to kill 51. Zhang B, Bowerman NA, Salama JK, et al. Induced
823 ^ 32. uveal melanoma cells. Clin Cancer Res 2007;13: sensitization of tumor stroma leads to eradication of
37. Attia P, Maker AV, Haworth LR, Rogers-Freezer L, 5219 ^ 25. established cancer by T cells. J Exp Med 2007;204:
Rosenberg, SA. Inability of a fusion protein of IL-2 and 44. Vlaykova T, Talve L, Hahka-Kemppinen M, et al. 49 ^ 55.
diphtheria toxin (Denileukin Diftitox, DAB389IL-2, Immunohistochemically detectable bcl-2 expression 52. Takimoto R, El-Deiry WS. Wild-type p53 transacti-
ONTAK) to eliminate regulatory T lymphocytes in in metastatic melanoma: association with survival vates the KILLER/DR5 gene through an intronic se-
patients with melanoma. J Immunother (1997) 2005; and treatment response. Oncology 2002;62:259 ^ 68. quence-specific DNA-binding site. Oncogene 2000;
28:582 ^ 92. 45. Oltersdorf T, Elmore SW, Shoemaker AR, et al. An 19:1735 ^ 43.
38. Insinga A, Minucci S, Pelicci PG. Mechanisms of inhibitor of Bcl-2 family proteins induces regression 53. Reits EA, Hodge JW, Herberts CA, et al. Radiation
selective anticancer action of histone deacetylase of solid tumours. Nature 2005;435:677 ^ 81. modulates the peptide repertoire, enhances MHC
inhibitors. Cell Cycle 2005;4:741 ^ 3. 46. Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 class I expression, and induces successful antitumor
39. Schumacher TN, Heemels MT, Neefjes JJ, et al. Di- antisense (oblimersen sodium) plus dacarbazine in immunotherapy. J Exp Med 2006;203:1259 ^ 71.
rect binding of peptide to empty MHC class I mole- patients with advanced melanoma: the Oblimersen Mela- 54. Parsa AT,Waldron JS, Panner A, et al. Loss of tumor
cules on intact cells and in vitro. Cell 1990;62:563 ^ 7. noma Study Group. J Clin Oncol 2006;24:4738 ^ 45. suppressor PTEN function increases B7-H1 expres-
40. Schumacher LY, Vo DD, Garban HJ, et al. Immuno- 47. Schimmer AD,Welsh K, Pinilla C, et al. Small-molecule sion and immunoresistance in glioma. Nat Med 2007;
sensitization of tumor cells to dendritic cell-activated antagonists of apoptosis suppressor XIAP exhibit broad 13:84 ^ 8.
immune responses with the proteasome inhibitor bor- antitumor activity. Cancer Cell 2004;5:25 ^ 35. 55. Yu H, Jove R. The STATs of cancerLnew molecular
tezomib (PS-341, Velcade). J Immunol 2006;176: 48. Franco AV, Zhang XD,Van Berkel E, et al. The role of targets come of age. Nat Rev Cancer 2004;4:97 ^ 105.
4757 ^ 65. NF-nB in TNF-related apoptosis-inducing ligand 56. Kortylewski M, Kujawski M,WangT, et al. Inhibiting
41. Nakata S, Yoshida T, Horinaka M, et al. Histone (TRAIL)-induced apoptosis of melanoma cells. Stat3 signaling in the hematopoietic system elicits
deacetylase inhibitors upregulate death receptor 5/ J Immunol 2001;166:5337 ^ 45. multicomponent antitumor immunity. Nat Med 2005;
TRAIL-R2 and sensitize apoptosis induced by TRAIL/ 49. SayersTJ, Brooks AD, Koh CY, et al.The proteasome 11:1314 ^ 21.
www.aacrjournals.org 4391 Clin Cancer Res 2008;14(14) July 15, 2008
Anda mungkin juga menyukai
- Melero 2007Dokumen12 halamanMelero 2007Django BoyeeBelum ada peringkat
- 2020 - Tun - HL ImmunotherapyDokumen10 halaman2020 - Tun - HL ImmunotherapyMiguel ÁngelBelum ada peringkat
- Pancreatic CancerDokumen9 halamanPancreatic Cancernski2104Belum ada peringkat
- Rycan 2021200134Dokumen6 halamanRycan 2021200134Sakkuru Yeyen LiviaBelum ada peringkat
- Cancer ModellingDokumen15 halamanCancer ModellingAzza Ahmed100% (1)
- Weiner 1Dokumen8 halamanWeiner 1Raúl ChoqueBelum ada peringkat
- A Paradigm Shift in Cancer Immunotherapy - From Enhancement To NormalizationDokumen23 halamanA Paradigm Shift in Cancer Immunotherapy - From Enhancement To NormalizationSWAGATIKA CHANDABelum ada peringkat
- Cancerimmunotherapies: Philip J. BergmanDokumen22 halamanCancerimmunotherapies: Philip J. BergmanRiefkyansyah PutraBelum ada peringkat
- Cancerimmunotherapies: Philip J. BergmanDokumen22 halamanCancerimmunotherapies: Philip J. BergmanLydia Angelia YanitaBelum ada peringkat
- Cancer Immunotherapy Beyond Checkpoint BlockadeDokumen16 halamanCancer Immunotherapy Beyond Checkpoint Blockaderaxi bacumabBelum ada peringkat
- Drake 2006Dokumen3 halamanDrake 2006Ruben NatarîșBelum ada peringkat
- 2.2. Cancer Immunotherapy Co-Stimulatory Agonists and Co-Inhibitory AntagonistsDokumen11 halaman2.2. Cancer Immunotherapy Co-Stimulatory Agonists and Co-Inhibitory AntagonistsDiego EskinaziBelum ada peringkat
- Marzagalli 2019Dokumen15 halamanMarzagalli 2019Wojciech WawrętyBelum ada peringkat
- Tumor MicroenvironmentDari EverandTumor MicroenvironmentPeter P. LeeBelum ada peringkat
- KCBT 18 07 1323596 PDFDokumen19 halamanKCBT 18 07 1323596 PDFFitria Rizky AmaliaBelum ada peringkat
- Artículo BaseDokumen10 halamanArtículo BaseSöö FiiBelum ada peringkat
- 18 JL 7Dokumen15 halaman18 JL 7Irma SihotangBelum ada peringkat
- Current Status and Future Directions of Cancer ImmunotherapyDokumen9 halamanCurrent Status and Future Directions of Cancer ImmunotherapyRo KohnBelum ada peringkat
- Reviews: Systemic Immunity in CancerDokumen15 halamanReviews: Systemic Immunity in Cancerliliana-contrerasBelum ada peringkat
- Wedekind2018 Article PediatricCancerImmunotherapyOpDokumen14 halamanWedekind2018 Article PediatricCancerImmunotherapyOpKAREN JAZMIN RUIZ MONROYBelum ada peringkat
- Biomedicines: Virus-Based Immuno-Oncology ModelsDokumen16 halamanBiomedicines: Virus-Based Immuno-Oncology ModelsAhana MukherjeeBelum ada peringkat
- Ca Immunotherapy Beyond PDFDokumen25 halamanCa Immunotherapy Beyond PDFJamil ABelum ada peringkat
- Inmunoterapia en Cancer de PulmonDokumen7 halamanInmunoterapia en Cancer de PulmonJorge ZegarraBelum ada peringkat
- 2021-Review-Tumour Neoantigen Mimicry by Microbial Species in Cancer ImmunotherapyDokumen11 halaman2021-Review-Tumour Neoantigen Mimicry by Microbial Species in Cancer ImmunotherapyCristian Felipe Sandoval QuiñonezBelum ada peringkat
- Tanyi 2012Dokumen15 halamanTanyi 2012Carlos ImasBelum ada peringkat
- Reprogramming The Tumour Microenvironment by RadioDokumen12 halamanReprogramming The Tumour Microenvironment by RadioCarlos N. Rojas PuyolBelum ada peringkat
- Avances en Nuevas Estrategias de Vacunas para La Inmunoterapia y Prevención Del Cáncer JAY A. BERZOFSKY 2004Dokumen11 halamanAvances en Nuevas Estrategias de Vacunas para La Inmunoterapia y Prevención Del Cáncer JAY A. BERZOFSKY 2004Ramiro J. Rodriguez GarciaBelum ada peringkat
- Cancer Immunology and ImmunotherapyDokumen15 halamanCancer Immunology and ImmunotherapyRamona PalalogosBelum ada peringkat
- Ru Ella 2013Dokumen25 halamanRu Ella 2013Ruben NatarîșBelum ada peringkat
- Cancers 12 03581 v2Dokumen25 halamanCancers 12 03581 v2Danna VeraBelum ada peringkat
- Immune Checkpointtargeted Therapy Cancer and Autoimmune Diseases Represent Two Sides of The Same CoinDokumen4 halamanImmune Checkpointtargeted Therapy Cancer and Autoimmune Diseases Represent Two Sides of The Same Coinpratiwi eka rahmawatiBelum ada peringkat
- Cancer and The Immune System Basic Concepts and Targets For InterventionDokumen38 halamanCancer and The Immune System Basic Concepts and Targets For InterventionMariana NannettiBelum ada peringkat
- Next Generation of Immune Checkpoint Therapy in Cancer: New Developments and ChallengesDokumen20 halamanNext Generation of Immune Checkpoint Therapy in Cancer: New Developments and ChallengesMarcelitaTaliaDuwiriBelum ada peringkat
- 4-Peptide Vaccine of The FutureDokumen12 halaman4-Peptide Vaccine of The Futureabdulloh suyutiBelum ada peringkat
- Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances The Ef Ficacy of Immune-Checkpoint BlockadeDokumen12 halamanTumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances The Ef Ficacy of Immune-Checkpoint BlockadeTaha AzadBelum ada peringkat
- Fonc 08 00086Dokumen14 halamanFonc 08 00086Siska HarapanBelum ada peringkat
- HHS Public Access: Cell-Intrinsic Barriers of T Cell-Based ImmunotherapyDokumen24 halamanHHS Public Access: Cell-Intrinsic Barriers of T Cell-Based Immunotherapyro111111Belum ada peringkat
- Immunotherapy in Uterine Cancers: January 2011Dokumen9 halamanImmunotherapy in Uterine Cancers: January 2011Nurul Ulya RahimBelum ada peringkat
- An Overview On Listeria Monocytogenes As A Versatile Vector For Cancer ImmunotherapyDokumen3 halamanAn Overview On Listeria Monocytogenes As A Versatile Vector For Cancer ImmunotherapyBIOMEDSCIDIRECT PUBLICATIONSBelum ada peringkat
- Lecture 3 Immunology of CancerDokumen4 halamanLecture 3 Immunology of CancerAishwarya SinghBelum ada peringkat
- Ascierto1 2013Dokumen4 halamanAscierto1 2013TrabelsiBelum ada peringkat
- Fast Facts: Managing Immune-Related Adverse Events in OncologyDari EverandFast Facts: Managing Immune-Related Adverse Events in OncologyBelum ada peringkat
- MDX 444Dokumen7 halamanMDX 444T HoranBelum ada peringkat
- CLINICS 2019 - Adoptive T-Cell Therapy For Solid MalignanciesDokumen15 halamanCLINICS 2019 - Adoptive T-Cell Therapy For Solid MalignanciesWilverCarbonelLuyoBelum ada peringkat
- The Future of Immune Checkpoint Therapy: Padmanee Sharma and James P. AllisonDokumen6 halamanThe Future of Immune Checkpoint Therapy: Padmanee Sharma and James P. Allisonlos mejores icfesBelum ada peringkat
- Intratumoral G100, A TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients With Merkel Cell CarcinomaDokumen11 halamanIntratumoral G100, A TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients With Merkel Cell Carcinomamasaladin3456789Belum ada peringkat
- Resistance in Prostate CancerDokumen16 halamanResistance in Prostate CancerMohammed AlabdullahBelum ada peringkat
- ICI in OncologyDokumen19 halamanICI in Oncologyraxi bacumabBelum ada peringkat
- Molecules: A Review On Cancer Immunotherapy and Applications of Nanotechnology To Chemoimmunotherapy of Different CancersDokumen24 halamanMolecules: A Review On Cancer Immunotherapy and Applications of Nanotechnology To Chemoimmunotherapy of Different CancersJuan Vera SanchezBelum ada peringkat
- +++kpwilkie Chapter RevisedDokumen35 halaman+++kpwilkie Chapter RevisedEnes ÇakmakBelum ada peringkat
- CAR T Cells For Brain Tumors: Lessons Learned and Road AheadDokumen25 halamanCAR T Cells For Brain Tumors: Lessons Learned and Road AheadFranco CalabiBelum ada peringkat
- Review Article: The Immune Response To Tumors As A Tool Toward ImmunotherapyDokumen12 halamanReview Article: The Immune Response To Tumors As A Tool Toward ImmunotherapyFrontiersBelum ada peringkat
- ImmunotherapyDokumen17 halamanImmunotherapyJonathan VenryBelum ada peringkat
- Aranda 2015Dokumen11 halamanAranda 2015TrabelsiBelum ada peringkat
- Paper 3Dokumen11 halamanPaper 3Ying-Chi ChuBelum ada peringkat
- Dendritic and CAR T Cell Therapy Against Cancer ReviewDokumen7 halamanDendritic and CAR T Cell Therapy Against Cancer ReviewAyushi MauryaBelum ada peringkat
- Edbk 237967Dokumen15 halamanEdbk 237967SYED MAAZ TARIQBelum ada peringkat
- Terapia Inmunomoduladora 1Dokumen9 halamanTerapia Inmunomoduladora 1Nathy Pasapera AlbanBelum ada peringkat
- Fast Facts: Managing immune-related Adverse Events in Oncology: Early recognition, prompt intervention, effective managementDari EverandFast Facts: Managing immune-related Adverse Events in Oncology: Early recognition, prompt intervention, effective managementBelum ada peringkat
- Natural Killer Cells in Cancer Biology and TherapyDokumen26 halamanNatural Killer Cells in Cancer Biology and Therapyhoangphuong08101992Belum ada peringkat
- Cancer TreatmentDokumen6 halamanCancer TreatmentJoshua DauzBelum ada peringkat
- Barrett 2003Dokumen21 halamanBarrett 2003小次郎 佐々木Belum ada peringkat
- Alternative ClinicsDokumen25 halamanAlternative ClinicsJimmi Jauhari HadiBelum ada peringkat
- Pharmacology TableDokumen9 halamanPharmacology TableMaryam KhushbakhatBelum ada peringkat
- A Reappraisal of CTLA-4 Checkpoint Blockade in Cancer ImmunotherapyDokumen17 halamanA Reappraisal of CTLA-4 Checkpoint Blockade in Cancer ImmunotherapyKRUBAKARAN MUTHUSAMYBelum ada peringkat
- Cancer Immunotherapy PowerpointDokumen32 halamanCancer Immunotherapy PowerpointsvswenBelum ada peringkat
- 3marchrice NewsDokumen56 halaman3marchrice NewsMujahid AliBelum ada peringkat
- Humanized Mice For The Study of Immuno-Oncology: ReviewDokumen16 halamanHumanized Mice For The Study of Immuno-Oncology: ReviewMaria Camila MejíaBelum ada peringkat
- Different Approaches To Atopic Dermatitis by Allergists, Dermatologists, and PediatriciansDokumen9 halamanDifferent Approaches To Atopic Dermatitis by Allergists, Dermatologists, and PediatriciansyelsiBelum ada peringkat
- Unseen Trends in Biotechnology-Part1Dokumen100 halamanUnseen Trends in Biotechnology-Part1Shourya17Belum ada peringkat
- Plasticity of Macrophage 2019 ReviewDokumen9 halamanPlasticity of Macrophage 2019 ReviewKudelko MatBelum ada peringkat
- 8 - Anti Allergic DrugsDokumen8 halaman8 - Anti Allergic DrugsMoataz TrabehBelum ada peringkat
- Traditional Medicinal Herbs For Healthiness and Fitness During TheDokumen13 halamanTraditional Medicinal Herbs For Healthiness and Fitness During TheTalitha Ulfah FakhiraBelum ada peringkat
- Ethacridinlactat Reinhardt Wounds 2005Dokumen10 halamanEthacridinlactat Reinhardt Wounds 2005Esti SaraswatiBelum ada peringkat
- Fungal Vaccines and ImmunotherapeuticsDokumen13 halamanFungal Vaccines and Immunotherapeuticshamza aliBelum ada peringkat
- Seminar On Cancer: Submitted To Submitted byDokumen13 halamanSeminar On Cancer: Submitted To Submitted byUdaya SreeBelum ada peringkat
- Understanding Cancer, Preventing It & Care If It HappensDokumen50 halamanUnderstanding Cancer, Preventing It & Care If It HappensShankar ShriramanujBelum ada peringkat
- Major Public Health & Lifestyle Issues in IndiaDokumen15 halamanMajor Public Health & Lifestyle Issues in IndiakomalBelum ada peringkat
- Cancers: MAIT Cells: Partners or Enemies in Cancer Immunotherapy?Dokumen23 halamanCancers: MAIT Cells: Partners or Enemies in Cancer Immunotherapy?János JuhászBelum ada peringkat
- Antimicrobial Implications of Vitamin D PDFDokumen11 halamanAntimicrobial Implications of Vitamin D PDFRizky Anindhia PutriBelum ada peringkat
- Allergen ImmunotherapyDokumen3 halamanAllergen ImmunotherapytimvrghsBelum ada peringkat
- A 60-Year-Old Female With Metastatic Malignant Melanoma of The Scalp Responding To IpilimumabDokumen4 halamanA 60-Year-Old Female With Metastatic Malignant Melanoma of The Scalp Responding To IpilimumabDyerik LilingBelum ada peringkat
- Group 5 - Immunotherapy and VaccineDokumen78 halamanGroup 5 - Immunotherapy and VaccineThe KingBelum ada peringkat
- NPC Immunotherapy (Corey Smith)Dokumen7 halamanNPC Immunotherapy (Corey Smith)fitrianyBelum ada peringkat
- Basic Principle of ChemotherapyDokumen29 halamanBasic Principle of ChemotherapyDevby Ulfandi100% (1)
- Medicinal Uses of Mushroom: Amit PrasadDokumen21 halamanMedicinal Uses of Mushroom: Amit PrasadAmit PrasadBelum ada peringkat
- Chapter 26Dokumen18 halamanChapter 26Chakravarthy PaBelum ada peringkat
- Crinum - An Endless Source of Bioactive Principles A Review - Part VDokumen14 halamanCrinum - An Endless Source of Bioactive Principles A Review - Part VPeter Tử Hà100% (1)
- Medical Ebook CollectionDokumen532 halamanMedical Ebook CollectionJasmine Agrabah100% (1)
- The Evolving Tumor Microenvironment From Cancer Initiation To Metastatic OutgrowthDokumen30 halamanThe Evolving Tumor Microenvironment From Cancer Initiation To Metastatic OutgrowthJose EdgarBelum ada peringkat