Light
Diunggah oleh
Tyba314Deskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Light
Diunggah oleh
Tyba314Hak Cipta:
Format Tersedia
The sun is probably the one thing we see most often throughout our lives.
Whenever we raise our sight to the sky during the day, we can see its dazzling light. If someone were to come up and ask "What good is the sun? we would probably reply without even a thought that the sun gives us light and heat. That answer, although a bit superficial, would be correct. But does the sun just "happen" to radiate light and heat for us? Is it accidental and unplanned? Or is the sun specially designed for us? Could this great ball of fire in the sky be a gigantic "lamp" that was created so as to meet our exact needs? Recent research indicates that the answer to the last two questions is "yes". "Yes" because in sunlight there is a design that inspires amazement.
between 0.70 microns and 0.40 microns and if you'd like to see it, you can: just raise your head and look around-it's called "visible light". This radiation causes chemical reactions to take place in your eyes and that is why you are able to see. The radiation known as "visible light" makes up 41% of sunlight even though it occupies less than 1/1025 of the whole electromagnetic spectrum. In his famous article "Life and Light", which appeared in Scientific American, the renowned physicist George Wald considered this matter and wrote "the radiation that is useful in prompting orderly chemical reactions comprises the great bulk of that of our sun." That the sun should radiate light so exactly right for life is indeed an extraordinary example of design.
The Right Wavelength
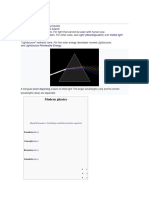
Both light and heat are different manifestations of electromagnetic radiation. In all its manifestations, electromagnetic radiation moves through space in waves similar to those created when a stone is thrown into a lake. And just as the ripples created by the stone may have different heights and the distances between them may vary, electromagnetic radiation also has different wavelengths. The analogy shouldn't be taken too far however because there are huge differences in the wavelengths of electromagnetic radiation. Some are several kilometers long while others are shorter than a billionth of a centimeter and the other wavelengths are to be found in a smooth, unbroken spectrum everywhere in between. To make things easier, scientists divide this spectrum up according to wavelength and they assign different names to different parts of it. The radiation with the shortest wavelength (one-trillionth of a centimeter) for example is called "gamma rays": these rays pack tremendous amounts of energy. The longest wavelengths are called "radio waves": they can be several kilometers long but carry very little energy. (One result of this is that radio waves are quite harmless to us while exposure to gamma rays can be fatal.) Light is a form of electromagnetic radiation that lies between these two extremes. The first thing to be noticed about the electromagnetic spectrum is how broad it is: the longest wavelength is 1025 times the size of the shortest one. Written out in full, 10 25 looks like this: 10,000,000,000,000,000,000,000,000 A number that big is pretty meaningless by itself. Let's make a few comparisons. For example, in 4 billion years (the estimated age of the earth) there are about 1017 seconds. If you wanted to count from 1 to 1025 and did so at the rate of one number a second nonstop, day and night, it would take you 100 million times longer than the age of the earth! ! If we were to build a pile of 1025 playing cards, we would end up with a stack stretching halfway across the observable universe. This is the vast spectrum over which the different wavelengths of the universe's electromagnetic energy extend. Now the curious thing about this is that the electromagnetic energy radiated by our sun is restricted to a very, very narrow section of this spectrum. 70% of the sun's radiation has wavelengths between 0.3 and 1.50 microns and within that narrow band there are three types of light: visible light, nearinfrared light, and ultraviolet light. Three kinds of light might seem quite enough but all three combined make up an almost insignificant section of the total spectrum. Remember our 1025
playing cards extending halfway across the universe? Compared with the total, the width of the band of light radiated by the sun corresponds to just one of those cards! Why should sunlight be limited to such a narrow range? The answer to that question is crucial because the only radiation that is capable of supporting life on earth is the kind that has wavelengths falling within this narrow range. In Energy and the Atmosphere, the British physicist Ian Campbell addresses this question and says "That the radiation from the sun (and from many sequence stars) should be concentrated into a minuscule band of the electromagnetic spectrum which provides precisely the radiation required to maintain life on earth is very remarkable." According to Campbell, this situation is "staggering". Nearly all of the sun's radiation is restricted to a narrow band of wavelengths ranging from 0.3 to 1.50 microns. This band encompasses near ultraviolet, visible, and infrared light. When we look at this part of the light we see that a large part of solar radiation falling outside the range of visible light is in the section of the spectrum called "near infrared". This begins where visible light ends and again occupies a very small part of the total spectrum-less than 1/1025. Is infrared light good for anything? Yes, but this time it's no use to look around because you can't see it with the naked eye. However you can easily feel it: the warmth you feel on your face when you look up on a bright sunny summer or spring day is caused by infrared radiation coming from the sun. The sun's infrared radiation is what carries the thermal energy that keeps Earth warm. It too is as essential for life as visible light is. And the fascinating thing is that our sun was apparently created just to serve for these two purposes, because these two kinds of light make up the greatest part of sunlight. And the third part of sunlight? Is that of any benefit? You can bet on it. This is "near ultraviolet light" and it makes up the smallest fraction of sunlight. Like all ultraviolet light, it is highly energized and it can cause damage to living cells. The sun's ultraviolet light however is the "least harmful" kind since it is closest to visible light. Although overexposure to solar ultraviolet light has been shown to cause cancer and cellular mutations, it has one vital benefit: the ultraviolet light concentrated in such a miniscule band is needed for the synthesis of vitamin D in humans and other vertebrates. (Vitamin D is necessary for the formation and nourishment of bone: without it, bones become soft or malformed, a disease called rickets that occurs in people deprived of sunlight for great lengths of time.) In other words, all the
From Ultraviolet to Infrared
We said that there was a range of 1:1025 in the sizes of the longest and shortest electromagnetic wavelengths. We also said that the amount of energy that was carried depended upon the wavelength: shorter wavelengths pack more energy than longer ones. Another difference has to do with how radiation at different wavelengths interacts with matter. The shortest forms of radiation are called (in increasing order of wavelength) "gamma rays", "X-rays", and "ultraviolet light". They have the ability to split atoms because they are so highly energized. All three can cause molecules-especially organic molecules-to break up. In effect, they tear matter apart at the atomic or molecular level. Radiation with wavelengths longer than visible light begins at infrared and extends up to radio waves. Its impact upon matter is less serious because the energy it conveys is not as great. The "impact upon matter" that we spoke of has to do with chemical reactions. A significant number of chemical reactions can take place only if energy is added to the reaction. The energy required to start a chemical reaction is called its "energy threshold". If the energy is less than this threshold, the reaction will never start and if it is more, it is of no good: in either case, the energy will have been wasted. In the whole electromagnetic spectrum, there is just one little band that has the energy to cross this threshold exactly. Its wavelengths range
radiation emitted by the sun is essential to life: none of it is wasted. The amazing thing is that all this radiation is limited to a 1/1025 interval of the whole electromagnetic spectrum yet it is sufficient to keep us warm, see, and allow all the chemical reactions necessary for life to take place. If the light radiated by the sun fell into any other part of the electromagnetic spectrum, there could be no life on Earth. It is certainly impossible to explain the fulfillment of this condition having a probability of 1 in 1025 with a logic of coincidence. And if all this were not enough, light does something else: it keeps us fed, too! Photosynthesis and Light First let's brush off our high-school chemistry and take a look at the formula for the photosynthesis reaction: 6H2O + 6CO2 + Sunlight --> C6H12O6 + 6O2 Translated into words this means: Water and carbon dioxide and sunlight produces glucose and oxygen. To be more exact what is happening in this chemical reaction is that six molecules of water (H2O) combine with six molecules of carbon dioxide (CO2) in a reaction that is energized by sunlight. When the reaction is complete, the result is a single molecule of glucose ( C 6H12O6), a simple sugar that is a fundamental element of nutrition-, and six molecules of gaseous oxygen (O2). The source of all nutriments on our planet, glucose contains a great deal of energy. Simple though this reaction may look, it is in fact incredibly complex. There is only one place where it occurs: in plants. The plants of this world produce the basic food for all living things. Every other living thing is ultimately nourished in one way or another by glucose. Herbivorous animals eat the plants themselves and carnivorous animals eat plants and/or other animals. Human beings are no exception: our energy is derived from the food we eat and comes from the same source. Every apple, potato, chocolate, or steak or anything else you eat is supplying you with energy that came from the sun. But photosynthesis is important for another reason. The reaction has two products: in addition to glucose, it also releases six molecules of oxygen. What's happening here is that plants are continuously cleaning up an atmosphere that is constantly being "polluted" by air-breathing creatures-human beings and animals. If plants didn't release oxygen, the oxygen-breathers would eventually use up all the free oxygen in the atmosphere and that would be the end of them. This marvelous chemical reaction, which has never been duplicated in any laboratory, is taking place deep in the grass you step on and in trees you may not even notice Could sunlight also be deliberately tailored for photosynthesis as well? Or are plants flexible enough so that they can perform the reaction no matter which kind of light reaches them? The mechanism of photosynthesis is initiated by the absorption of sunlight by a chlorophyll molecule. But in order for this to occur, the light must be of the right color. Light of the wrong color won't do the trick. A good analogy is that of a television set. In order for the set to receive a given channel it must be tuned to that channel;
By the Sun and its Brightness
(Quran: 91:1)
tune it differently and the reception will not occur. It is the same with photosynthesis, the Sun functioning as the transmitter in the analogy and the chlorophyll molecule as the receiving TV set. If the molecule and the Sun are not tuned to each other-tuned in the sense of colour- photosynthesis will not occur. As it turns out, the sun's color is just right. One might think that a certain adaptation has been at work here: the adaptation of plant life to the properties of sunlight. After all, if the Sun were a different temperature could not some other molecule, tuned to absorb light of a different colour, take the place of chlorophyll? Remarkably enough the answer is no, for within broad limits all molecules absorb light of similar colours. The absorption of light is accomplished by the excitation of electrons in molecules to higher energy states, and the same no matter what molecule you are discussing. As things stand in reality, there is a good fit between the physics of stars and that of molecules. Failing this fit, however, life would have been impossible. The harmony between stellar and molecular physics is a harmony too extraordinary ever to be explained by chance. There was only one chance in 1025 of the sun's providing just the right kind of light necessary for us and that there should be molecules in our world that are capable of using that light. This perfect harmony is unquestionably proof of intentional, deliberate design. In other words, there is a single Creator, the Ruler of starlight and of the molecules of plants Who has created all these things in harmony with one other. Clearly, its a significant thing that Allah swears by Sun and its brightness in Surat Al-Shams (The Quran, ch.91), but even more He continues to swear with 7 more things to confirm a specific point. The first 7 ayat is the longest swear by Allah in the whole Quran! Isnt it time for you to find what is that point?? ____________________________________________________ For more info: www.islamindex.com
Design in Light
Why did God swear with the brightness of the sun?
Anda mungkin juga menyukai
- The Declaration of FaithDokumen57 halamanThe Declaration of FaithBooks for IslamBelum ada peringkat
- How and Wonder Book of RadiationDokumen35 halamanHow and Wonder Book of Radiationkett8233100% (4)
- X-Ray Free Electron Lasers: Principles, Properties and ApplicationsDokumen16 halamanX-Ray Free Electron Lasers: Principles, Properties and ApplicationsSarah JordanBelum ada peringkat
- Liquid Penetrant Inspection: World Centre For Materials Joining TechnologyDokumen69 halamanLiquid Penetrant Inspection: World Centre For Materials Joining TechnologyNowshad NowsaBelum ada peringkat
- IMCD B PC Embracing BeautyDokumen41 halamanIMCD B PC Embracing BeautyFarrensyah PutraBelum ada peringkat
- ASTM D 1148 - 95 (Reapproved 2001) Rubber Deterioration-Heat and Ultraviolet LightDokumen5 halamanASTM D 1148 - 95 (Reapproved 2001) Rubber Deterioration-Heat and Ultraviolet Lightalin2005Belum ada peringkat
- Introduction To Electromagnetic WavesDokumen11 halamanIntroduction To Electromagnetic WavesDharshana pjr100% (1)
- Effects of Electromagnetic Radiation To Living Things and EnvironmentDokumen15 halamanEffects of Electromagnetic Radiation To Living Things and EnvironmentGeorcelle CarcillarBelum ada peringkat
- Dealers Guide To Chemical Restoration of Postal StampsDokumen40 halamanDealers Guide To Chemical Restoration of Postal Stampspeter smith100% (2)
- Ophthalmic Dispensing Revision Guide: First Year Part OneDari EverandOphthalmic Dispensing Revision Guide: First Year Part OnePenilaian: 4 dari 5 bintang4/5 (3)
- Tinosorb's BrochureDokumen24 halamanTinosorb's Brochurelepnit304Belum ada peringkat
- Bearded Dragon Care SheetDokumen4 halamanBearded Dragon Care SheetJonathan 'Andy' TanBelum ada peringkat
- Concept of God in Major World Religions - Dr. Zakir NaikDokumen29 halamanConcept of God in Major World Religions - Dr. Zakir NaikArshad Farooqui100% (5)
- 1.1 Electromagnetic SpectrumDokumen17 halaman1.1 Electromagnetic SpectrumShreyas SvBelum ada peringkat
- Light: Electromagnetic Radiation Light (Disambiguation) Visible Light (Disambiguation)Dokumen19 halamanLight: Electromagnetic Radiation Light (Disambiguation) Visible Light (Disambiguation)AnandBelum ada peringkat
- The Illuminating World of Light with Max Axiom, Super Scientist: 4D An Augmented Reading Science ExperienceDari EverandThe Illuminating World of Light with Max Axiom, Super Scientist: 4D An Augmented Reading Science ExperienceBelum ada peringkat
- SpectrosDokumen21 halamanSpectrosAman Rathore100% (1)
- This Is The ProphetDokumen22 halamanThis Is The Prophetapi-25980564Belum ada peringkat
- EASA 66 Module 9 MCQDokumen3 halamanEASA 66 Module 9 MCQHamzeh Al-Qaisi100% (3)
- Woven Orchard Protection Crop Cover SpecificationDokumen18 halamanWoven Orchard Protection Crop Cover SpecificationVinay Tripathi100% (1)
- Experiment 8: Ultraviolet Examination: I. ObjectivesDokumen4 halamanExperiment 8: Ultraviolet Examination: I. ObjectivesMikhaela Andree MarianoBelum ada peringkat
- LightDokumen2 halamanLightapi-25980564Belum ada peringkat
- The Electromagnetic Spectrum Intro Web TaskDokumen4 halamanThe Electromagnetic Spectrum Intro Web Taskapi-308495066Belum ada peringkat
- Sia83 3notesDokumen5 halamanSia83 3notesAmaechi ZalBelum ada peringkat
- Electromagnetic Waves General Science LessonDokumen3 halamanElectromagnetic Waves General Science LessonNarahmie RuadoBelum ada peringkat
- What is Visible Light? Understanding the Spectrum of Light WavesDokumen9 halamanWhat is Visible Light? Understanding the Spectrum of Light WavesJF BatucalBelum ada peringkat
- Fundamental Law of LightsDokumen14 halamanFundamental Law of LightsJonel Juaneza100% (1)
- Gamma Rays: These Are The Most Energetic and Dangerous Form of ElectromagneticDokumen4 halamanGamma Rays: These Are The Most Energetic and Dangerous Form of ElectromagneticmubashirBelum ada peringkat
- BaboDokumen13 halamanBaboZezo EdrisBelum ada peringkat
- 2 - Pieces of EvidenceDokumen27 halaman2 - Pieces of EvidenceChosen ExeBelum ada peringkat
- Ce319 AssignmentDokumen9 halamanCe319 Assignmenttoba ziaeeBelum ada peringkat
- PhysicsDokumen20 halamanPhysicsMelanie De SousaBelum ada peringkat
- Light SeminarDokumen9 halamanLight Seminarvarsham2Belum ada peringkat
- Basics of Electromagnetic RadiationDokumen12 halamanBasics of Electromagnetic RadiationJoseph NyabugaBelum ada peringkat
- The Electromagnetic Spectrum by Cindy GriggDokumen2 halamanThe Electromagnetic Spectrum by Cindy GriggMae Dil G. Tirariray - PaladBelum ada peringkat
- Unit-2-Solar Thermal EnergyDokumen15 halamanUnit-2-Solar Thermal EnergyDr. Navneet Kumar PandeyBelum ada peringkat
- Review of LiteratureDokumen5 halamanReview of LiteratureAllen HabibovicBelum ada peringkat
- The Electromagnetic SpectrumDokumen15 halamanThe Electromagnetic Spectrumcindy lue parasBelum ada peringkat
- Life Giving Sun Written Report 1Dokumen10 halamanLife Giving Sun Written Report 1Jeraldine ManipolBelum ada peringkat
- Solar Radiation: Sunlight and More: Non Conventional Sources OF EnergyDokumen15 halamanSolar Radiation: Sunlight and More: Non Conventional Sources OF EnergyAnutosh BhaskarBelum ada peringkat
- Gen. Physics 2Dokumen49 halamanGen. Physics 2Shelamae MangyaoBelum ada peringkat
- Wave Nature of Light: Basic Properties of Waves: Amplitude, Wavelength, and FrequencyDokumen4 halamanWave Nature of Light: Basic Properties of Waves: Amplitude, Wavelength, and FrequencyJaden MoniezBelum ada peringkat
- Waves Reading PassageDokumen2 halamanWaves Reading PassagedavidBelum ada peringkat
- Electromagnetic Spectrum ReadingDokumen2 halamanElectromagnetic Spectrum Readingapi-369452118Belum ada peringkat
- Visible Light ExplainedDokumen5 halamanVisible Light ExplainedJef PerezBelum ada peringkat
- Environmental Science: What Is Environmental Physics?Dokumen5 halamanEnvironmental Science: What Is Environmental Physics?AtifMuhammadBelum ada peringkat
- Agro-Meteorology For BSC Agriculture StudentsDokumen54 halamanAgro-Meteorology For BSC Agriculture StudentsMadan ThapaBelum ada peringkat
- Chemistry Investigatory Project 12 BDokumen20 halamanChemistry Investigatory Project 12 Banitta0% (1)
- Uses of Electromagnetic WavesDokumen4 halamanUses of Electromagnetic WavesMarlou PrincesaBelum ada peringkat
- Grade 10 ReviewerDokumen6 halamanGrade 10 ReviewerDino M PangilinanBelum ada peringkat
- Chapter 3: Electromagnetic Waves - Radiant Energy II: Goals of Period 3Dokumen12 halamanChapter 3: Electromagnetic Waves - Radiant Energy II: Goals of Period 3iprateekBelum ada peringkat
- Lesson 2-Solar RadiationDokumen78 halamanLesson 2-Solar RadiationZyra Yvonne MangligotBelum ada peringkat
- Chem Atmosphere Notes StrtedDokumen3 halamanChem Atmosphere Notes StrtednabilahBelum ada peringkat
- Radiant Energy & Nuclear EnergyDokumen42 halamanRadiant Energy & Nuclear EnergyChristine Marielle Claroniño ApoBelum ada peringkat
- The Electromagnetic SpectrumDokumen5 halamanThe Electromagnetic SpectrumSamantha AmoreBelum ada peringkat
- Solar Radiation: Department of Physics and Astronomy, The Ohio State University, Columbus 10Dokumen14 halamanSolar Radiation: Department of Physics and Astronomy, The Ohio State University, Columbus 10Fawziah NasserBelum ada peringkat
- The Physics of LightDokumen6 halamanThe Physics of LightprashantsheetalBelum ada peringkat
- SCH 100 Notes 2013-2014 ALLDokumen42 halamanSCH 100 Notes 2013-2014 ALLErick Wangila WanyonyiBelum ada peringkat
- What is Light - An Overview of Properties in <40 CharsDokumen3 halamanWhat is Light - An Overview of Properties in <40 CharsLeo CerenoBelum ada peringkat
- ChemDokumen15 halamanChemSachin RaiBelum ada peringkat
- Solar Energy: Made By-Archit Tantia Aayush Tainwala Chandrakant SardaDokumen26 halamanSolar Energy: Made By-Archit Tantia Aayush Tainwala Chandrakant SardaArchitBelum ada peringkat
- CBSE XII Chemistry/Physics Project Spectroscopy and Its ApplicationsDokumen21 halamanCBSE XII Chemistry/Physics Project Spectroscopy and Its ApplicationsshantanurBelum ada peringkat
- Space Weather Question SolutionDokumen9 halamanSpace Weather Question Solutionbelinda abigaelBelum ada peringkat
- Solar Radiation SpectrumDokumen2 halamanSolar Radiation SpectrumAndres RojasBelum ada peringkat
- Ultraviolet: "UV" Redirects Here. For Other Uses, See - For Other Uses For "Ultraviolet", SeeDokumen2 halamanUltraviolet: "UV" Redirects Here. For Other Uses, See - For Other Uses For "Ultraviolet", SeeGemma CanicosaBelum ada peringkat
- LIGHT Resource GuideDokumen25 halamanLIGHT Resource Guidecourtney_fernan8968Belum ada peringkat
- Accelerated MotionDokumen20 halamanAccelerated MotionAdelina LiwayanBelum ada peringkat
- Astronomy QuizDokumen2 halamanAstronomy QuizAnnelise FrankBelum ada peringkat
- Chemistry Investigatory Project: Name: Yogesh Singh Class: Xii B Submitted To: Ms - Sharaddha NarwariyaDokumen36 halamanChemistry Investigatory Project: Name: Yogesh Singh Class: Xii B Submitted To: Ms - Sharaddha NarwariyaSinghnarwariyaBelum ada peringkat
- WakafatDokumen15 halamanWakafatTyba314Belum ada peringkat
- @@@@@@@@ @PB Jó at Õbîjãþa@ - Ó@åß@Dokumen23 halaman@@@@@@@@ @PB Jó at Õbîjãþa@ - Ó@åß@Tyba314Belum ada peringkat
- Walking The Straight Path Constancy, Renewal & The ContemporaryDokumen15 halamanWalking The Straight Path Constancy, Renewal & The Contemporaryapi-25885370Belum ada peringkat
- Seven Repeated Verses of QuranDokumen16 halamanSeven Repeated Verses of QuranhaiderknpcBelum ada peringkat
- American QuestionsDokumen47 halamanAmerican QuestionsAbdullah ElNaser Helmy عبدالله الناصر حلمىBelum ada peringkat
- Pitfalls in The Quest For Knowledge - ProofreadDokumen20 halamanPitfalls in The Quest For Knowledge - Proofreadapi-3709184Belum ada peringkat
- Amerca What We Dont KnowDokumen22 halamanAmerca What We Dont KnowAbdullah ElNaser Helmy عبدالله الناصر حلمىBelum ada peringkat
- How To Learn IslamDokumen58 halamanHow To Learn IslamAbdullah ElNaser Helmy عبدالله الناصر حلمىBelum ada peringkat
- Had at HaDokumen58 halamanHad at HaTyba314Belum ada peringkat
- Become Acquainted With IslamDokumen171 halamanBecome Acquainted With Islamlove75Belum ada peringkat
- The Meaning of Our Testimony That Muhammad (Peace Be Upon Him) Is The Messenger of AllahDokumen42 halamanThe Meaning of Our Testimony That Muhammad (Peace Be Upon Him) Is The Messenger of Allahislamiq100% (3)
- Thus Taught The Prophets: S H A y K H S A L M A N A L - A W D A HDokumen30 halamanThus Taught The Prophets: S H A y K H S A L M A N A L - A W D A Hapi-25885370Belum ada peringkat
- Personality Development in Islam and Its Effects On Nations and CivilizationsDokumen11 halamanPersonality Development in Islam and Its Effects On Nations and CivilizationsSHAMSUDDINBelum ada peringkat
- The Prophetic Commentary of The QuranDokumen23 halamanThe Prophetic Commentary of The Quranapi-3701716Belum ada peringkat
- Islam and SecularismDokumen14 halamanIslam and SecularismISLAMICULTUREBelum ada peringkat
- Islamic HomeDokumen13 halamanIslamic HomeadeebBelum ada peringkat
- Who Should Perform Ijtihâd? A Discussion On Independent JuristicDokumen27 halamanWho Should Perform Ijtihâd? A Discussion On Independent Juristicapi-25885370Belum ada peringkat
- Discourses On Islamic Law and Matters of Public ConcernDokumen18 halamanDiscourses On Islamic Law and Matters of Public Concernapi-25885370Belum ada peringkat
- Do So (Hajj)Dokumen36 halamanDo So (Hajj)Tyba314Belum ada peringkat
- 20 Ways To Show Off by Sheikh Salman B. Fahd Al-OadahDokumen27 halaman20 Ways To Show Off by Sheikh Salman B. Fahd Al-Oadahadeshs19852415Belum ada peringkat
- Jesus Christ The Son of Mary: Al-H.abīb Alī Al-JafrīDokumen15 halamanJesus Christ The Son of Mary: Al-H.abīb Alī Al-JafrīmosahmedBelum ada peringkat
- FactDokumen45 halamanFactmianabdulBelum ada peringkat
- Islamic QuestionnaireDokumen14 halamanIslamic QuestionnaireSaqib IqbalBelum ada peringkat
- The Purpose of LifeDokumen7 halamanThe Purpose of LifeTyba314Belum ada peringkat
- Why BeardDokumen4 halamanWhy Beardapi-25980564100% (1)
- Jesus Christ The Son of Mary: and His Most Blessed MotherDokumen36 halamanJesus Christ The Son of Mary: and His Most Blessed MotherTyba314Belum ada peringkat
- Who Wrote The QuranDokumen26 halamanWho Wrote The Quranrahman_camBelum ada peringkat
- Applications of gas discharge plasmas for surface modifications and lightingDokumen17 halamanApplications of gas discharge plasmas for surface modifications and lightingnewfolder20082008Belum ada peringkat
- Photoinitiators in Dentistry: A ReviewDokumen4 halamanPhotoinitiators in Dentistry: A ReviewmarishaBelum ada peringkat
- 06 Fab PDFDokumen1 halaman06 Fab PDFmarceloestimuloBelum ada peringkat
- Coatings, Resins, Pigments & Inks TechnologiesDokumen15 halamanCoatings, Resins, Pigments & Inks TechnologiesPravin Tandel100% (1)
- Optical PhenomenaDokumen23 halamanOptical PhenomenaMundu Mustafa100% (1)
- Pterygium - A Practical Guide To Management - 9788184487251Dokumen132 halamanPterygium - A Practical Guide To Management - 9788184487251mono1144Belum ada peringkat
- Thesis On Doped ZnoDokumen6 halamanThesis On Doped Znoameliarichardsonsouthbend100% (1)
- Cappa-Enit12-r01-A4fr SM Ez Eur BioairDokumen187 halamanCappa-Enit12-r01-A4fr SM Ez Eur Bioairaldo giovBelum ada peringkat
- Photochromic Materials: More Than Meets The EyeDokumen23 halamanPhotochromic Materials: More Than Meets The EyesanBelum ada peringkat
- Bactericidal Effects of Ozone in AirDokumen15 halamanBactericidal Effects of Ozone in AirJose BecerrilBelum ada peringkat
- Sterilization in OrthodonticsDokumen121 halamanSterilization in OrthodonticsSwati PawarBelum ada peringkat
- TDS of GalShield UV Care PLUSDokumen4 halamanTDS of GalShield UV Care PLUSDr. Rajesh Kumar SinghBelum ada peringkat
- Comprehensive Analysis of Effects of Ultraviolet Radiation Exposure On Grade 12 SMAW Students During The SMAW ProcessDokumen13 halamanComprehensive Analysis of Effects of Ultraviolet Radiation Exposure On Grade 12 SMAW Students During The SMAW ProcessHaru MitsuBelum ada peringkat
- Stability TestingDokumen3 halamanStability TestingSebabrata Ghosh DastidarBelum ada peringkat
- En c7012 Uv Flame Detector Handbook 60 2398 Nl05r0896Dokumen20 halamanEn c7012 Uv Flame Detector Handbook 60 2398 Nl05r0896mohamedBelum ada peringkat
- Surgery 8th Se Skin Subcu Compiled TransbooksnotestpreviewersDokumen237 halamanSurgery 8th Se Skin Subcu Compiled TransbooksnotestpreviewersMildred DagaleaBelum ada peringkat
- The EM Spectrum in 40 CharactersDokumen11 halamanThe EM Spectrum in 40 CharactersMarilyn LaquindanumBelum ada peringkat
- Manual CHEMIDOC PDFDokumen170 halamanManual CHEMIDOC PDFSheron CogoBelum ada peringkat
- PHS 102Dokumen11 halamanPHS 102Nafiu YunusaBelum ada peringkat
- Uwe Tutti FruttiDokumen16 halamanUwe Tutti FruttiPaulmanke33% (3)
- How To Observe The SunDokumen3 halamanHow To Observe The Sunnkvd134Belum ada peringkat