Pathophysiology / Pharmacology: Cardiac Physiology
Diunggah oleh
jazzdoc007Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Pathophysiology / Pharmacology: Cardiac Physiology
Diunggah oleh
jazzdoc007Hak Cipta:
Format Tersedia
1
Pathophysiology / Pharmacology
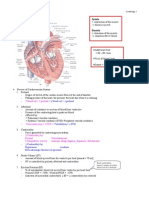
Cardiovascular Notes Cardiac Physiology The development of a working knowledge of cardiovascular pathophysiology and pharmacology is dependent upon a foundational understanding of normal cardiovascular anatomy and physiology. The better one can accurately visualize the dynamic processes that concomitantly occur during the cardiac cycle, the easier it will be to appreciate the effect of any abnormal intervention on the system as a whole. While it is appropriate to compartmentalize the events occurring in different anatomic locations as one is initially introduced to these phenomena, familiarity and comprehension should eventually allow one to appreciate the manner with which these specific processes contribute to overall cardiovascular function. In other words, what initially appears like numerous individual and separate trees on close observation, is in reality an integrated forest when viewed from a holistic perspective. As one is introduced to cardiovascular pharmacology and the specific drugs that are employed in the treatment of the major cardiovascular disorders, it will become apparent that these agents are designed to affect individual physiologic parameters in specific ways. What follows is a discussion of these individual physiologic principles, how they are affected by disease and how they are pharmacologically manipulated in an attempt to restore cardiovascular function. Preload and Contractility Preload can be generally be defined as the load or force applied to a system prior to work being done. In cardiovascular physiology, preload refers to the degree of stretch applied to an individual myocardial cell prior to ventricular contraction. The importance of the effect that a change in preload will have on ventricular contraction can be best appreciated with an understanding of the Frank Starling law of the heart. The Frank Starling law of the heart describes a direct relationship between the precontraction stretch of a myocyte and the force generated upon contraction of the muscle cell. Comprehension of this principle is facilitated by comparing the similarity between the actin and myosin fibers in a muscle cell and a metal spring. Applying stretch to both the spring and the actin / myosin structure by pulling at both ends increases the length of both of these systems. In physical terms, this increases the potential energy of both the spring and the muscle fibrils. When the ends are released, both the spring and the actin / myosin complex rapidly shorten back to their resting length. The force generated by this shortening (or contraction) is directly related to the degree to which they were lengthened by pulling at both ends. Plotting the strength of contraction (known as contractility) against the degree of stretch prior to contraction produces a graphic representation of the Frank Starling law of the heart: Paul Kelner, M.D.
On this diagram, it can be seen that the X axis plots preload and the Y axis plots stroke volume. As noted above, the X axis represents myocardial stretch. Visualizing the ventricle as a container of fluid that can expand as needed is helpful in appreciating the relationship between the volume of blood in the ventricle and the associated stretch of the muscular wall. Therefore, the volume of blood in the ventricle just prior to systole (called the End-Diastolic Volume or EDV) depends on how much blood is returned to the heart from the venous system and represents the Preload. As the preload volume changes, so does the degree to which the individual muscle cells stretch. In turn, the contractile force produced by the myocytes (contractility) is directly related to this stretch. The Y axis on the diagram above is labeled Stroke Volume. This represents the actual volume of blood ejected during systolic contraction and is a direct reflection of contractility. Summarizing and simplifying the concepts presented above: PRELOAD = End - Diastolic Volume (EDV) CONTRACTILITY represents the force of contraction associated with a specific amount of myocardial stretch prior to contraction. The FRANK - STARLING curve represents the relationship between PRELOAD and CONTRACTILITY The STROKE VOLUME represents the actual volume of blood ejected from the ventricle during systole
3 Further evaluation of the graph above reveals that at some degree of stretch there ceases to be an increase in contractility. This is analogous to the point at which a metal spring is stretched so greatly that the metal structure is damaged and it can not return to its original length. In a myocardial cell, this is when the actin / myosin complex is stretched to the point of protein damage and loses its elasticity. Afterload In mechanical terms, the afterload represents the resistance of the system that work is being done into. From a cardiovascular standpoint this is represented by the arterial blood pressure. The actual blood pressure is a function of the volume of blood in the vessel and the elasticity (or compliance) of the vessel wall. During ventricular systole, the ventricular myocardium contracts and ejects a volume of blood (stroke volume (SV)) into the pulmonary artery on the right and the aorta on the left. The relative effort that the ventricle must exert to push this blood into these vessels is directly dependent upon the ability of the vessels to receive this blood. The higher the resistance, the more work that the myocardium must exert to achieve a given stroke volume. Chronic afterload elevation often results in hypertrophy of the ventricular myocardium. While the actual mass of muscle is increased in the hypertrophic ventricle, the pumping action is decreased. The end result of this scenario is frequently associated with the development of CHF manifested by decreased exercise tolerance, shortness of breath and an overall diminution of the patients overall quality of life. Thus, in patients exhibiting persistently elevated blood pressure (increased afterload), early and aggressive intervention is often the recommended course of action. Ejection Fraction and Cardiac Output Even in individuals with excellent cardiac function, pumping efficiency does not come close to 100%. Regarding human cardiac mechanical efficiency, calculation of the Ejection Fraction (stroke volume / end-diastolic volume) gives clinicians a quantitative and objective parameter that can be used to compare a patients cardiac function with that of other individuals of similar circumstances and demographics. This value can also be useful in quantifying a patients cardiovascular function over time. Despite the straightforward mathematic calculation, determining a patients ejection fraction (EF) can not be done without some form of imaging. Obviously, the actual end diastolic volume and stroke volume are not readily obtainable at the bedside. To measure these volumes, patients frequently undergo echocardiographic imaging. This non invasive ultrasonic study is done routinely and provides a significant amount of information regarding cardiovascular structure and function. Besides measuring the EF, EDV and SV, information related to valvular function, atrial and ventricular volumes and myocardial wall movement can be discerned. Another cardiac parameter that is useful in assessing a patients cardiac function is the Cardiac Output (CO). This value measures the volume of blood pumped by the heart per unit of time. It is calculated by multiplying the heart rate (HR) and the SV. Expressed formulaically, it appears as follows:
Cardiac Output (CO) = Heart Rate (HR) x Stroke Volume (SV) In humans, the CO is most often expressed as liters / minute. A typical value for the cardiac output is 5 liters / minute. Similarly to the EF, calculation of the CO requires determination of a patients SV. Currently, the CO can be obtained using echocardiography, angiography (cardiac catheterization) and / or via a specialized cardiac catheter called a Swan Ganz catheter. Maintenance and Regulation of Arterial Blood Pressure Arterial blood pressure (afterload) is regulated by both the central nervous system and the endocrine system. From a neurologic standpoint, the baroreceptor reflex is the major structure that senses changes in blood pressure. The baroreceptors themselves are primarily located near both carotid arteries and also the thoracic aorta. These structures are very sensitive to changes in pressure in these large arteries. Changes in BP stimulate the receptors to send neuronal messages to primarily brainstem components, such as the medulla and the pons. When these signals are received, these CNS structures initiate many physiologic responses in an attempt to correct the change in blood pressure. An increase in BP results in generalized arterial vasodilatation and a corresponding decrease in HR. As noted above, the HR is one of the factors directly contributing to the cardiac output. Therefore, the decrease in HR and the associated arterial vasodilatation results in a decrease in BP, thus compensating for the increase in BP that initiated this reflex. Conversely, a decrease in arterial BP results in vasoconstriction and an increase in HR, producing a compensatory elevation of BP. As noted previously, the baroreceptors are very sensitive to local changes in pressure. Those providing patient care need to be cognizant of this fact, as the application of sufficient external pressure, such as that applied when palpating the carotid pulse, can result in a syncopal reaction by the patient. The medullary signals leading to the decrease in HR and vasodilatation, in response to an increase in BP or pressure applied externally, are transmitted via parasympathetic impulses carried by branches of the tenth cranial (vagus) nerve. This is the origin of the frequent slang verb vagaled that is used when describing a syncopal reaction in a patient. Fortunately, most patients who lose consciousness secondary to this mechanism respond to being placed in a supine position and placing a cold compress on the forehead.
The Baroreceptor Reflex
As noted above, arterial BP is regulated by both the nervous system and the endocrine system. Endocrine regulation of fluid volume and blood pressure is complex and involves the coordinated action of many individual systems. One of the primary hormonal systems involved in the regulation of BP is the rennin angiotensin aldosterone system (RAAS). The primary goal of this system is the maintenance of the glomerular filtration rate (GFR) in the kidneys. In order for effective filtration to occur in the nephron, the hydrostatic pressure of the blood in the glomerulus must be sufficient to overcome the oncotic pressure produced by protein and cells. As with any filtration system, the pressure of the fluid that is being filtered must be high enough to force the fluid through the sieve or filter. Renal dysfunction, secondary to insufficient filtration pressure, will result in the build up of toxins in the blood and abnormalities in electrolyte concentration. These events can result in significant morbidity and/or mortality acutely, thus justifying the need for sensitive and effective physiologic control. A drop in hydrostatic pressure, sufficient to decrease GFR, results in the release of rennin by the juxta-glomerular cells, which are located near Bowmans capsule. Rennin enters the general circulation and acts to convert the pro-hormone, angiotensinogen into angiotensin I. This form of angiotensin is physiologically inactive. However, the physiologically insignificant angiotensin I is converted into angiotensin II by the enzyme called angiotensin converting enzyme (ACE). This form of angiotensin has significant physiologic effects. It results in marked arterial vasoconstriction and stimulates the release of aldosterone from the adrenal cortex. Aldosterone then acts at the level of the kidney to increase sodium retention in the nephron. As sodium levels are increased in the serum, free water follows, secondary to osmotic pressure. The overall effect of both of these actions is to increase intra-vascular volume and arterial blood pressure. In healthy individuals, these events will lead to an increase in the GFR, thus achieving the primary goal of this system. It should be noted, however, that in many patients, the initial decrease in GFR occurred secondary to inadequate renal perfusion, resulting from renal arterial atherosclerosis. In these patients, the action of the RAAS results in the development of significant HTN, and additionally fails to improve GFR, as the elevation of BP cannot overcome the obstruction to blood flow caused by the atherosclerotic plaques.
The Rennin-Angiotensin-Aldosterone System Arteriosclerosis
Renal Artery Atherosclerosis
The term arteriosclerosis is a general term for any pathological process that results in an overall hardening of the arterial wall. There are many such processes some common and others rarely seen. By a significant margin, the most prevalent of these is atherosclerosis. From a global perspective, both anatomically and geographically, this disease is responsible for a major percentage of all human morbidity and mortality, and results in billions of dollars spent toward research, treatment and job related losses. The risk factors and lifestyle issues that contribute to this disease process are, in general, well known and include genetics, diet, smoking, lack of exercise, diabetes mellitus and several others, which are less well defined.
Anda mungkin juga menyukai
- Central Venous Pressure: Its Clinical Use and Role in Cardiovascular DynamicsDari EverandCentral Venous Pressure: Its Clinical Use and Role in Cardiovascular DynamicsBelum ada peringkat
- Cardiovascular and Autonomic Influences On Blood Pressure: John E. Jones,, Aruna R. Natarajan,,, and Pedro A. JoseDokumen23 halamanCardiovascular and Autonomic Influences On Blood Pressure: John E. Jones,, Aruna R. Natarajan,,, and Pedro A. JoseLulu LuwiiBelum ada peringkat
- Hemodynamics For The Bedside Nurse 1CEUDokumen7 halamanHemodynamics For The Bedside Nurse 1CEURN333100% (1)
- Heart-Lung Interaction in Spontaneous Breathing Subjects: The BasicsDokumen9 halamanHeart-Lung Interaction in Spontaneous Breathing Subjects: The BasicsRaniBelum ada peringkat
- CV PathoDokumen32 halamanCV PathoSaif AliBelum ada peringkat
- Icu BookDokumen1.054 halamanIcu BookqsychoBelum ada peringkat
- Funk2013 1Dokumen7 halamanFunk2013 1BiancaPancuBelum ada peringkat
- Hypertension: A Comparative Review Based On Fractal Wave Theory of ContinuumDokumen8 halamanHypertension: A Comparative Review Based On Fractal Wave Theory of ContinuumAdaptive MedicineBelum ada peringkat
- Cardiac Physiology Dissertation IdeasDokumen6 halamanCardiac Physiology Dissertation IdeasBuyAPhilosophyPaperUK100% (2)
- Diastolic Dysfunction in Heart Failure Review LEEERRRDokumen18 halamanDiastolic Dysfunction in Heart Failure Review LEEERRRchris chavezBelum ada peringkat
- 171 FullDokumen5 halaman171 FullOdrig De LavalleBelum ada peringkat
- Wiki Pressure-Volume Loop Analysis in CardiologyDokumen11 halamanWiki Pressure-Volume Loop Analysis in Cardiologydgina8800Belum ada peringkat
- 22cardiac PV Loop Cardiac PhysiologyDokumen19 halaman22cardiac PV Loop Cardiac PhysiologyJaydave PatelBelum ada peringkat
- Cardiovascular Changes in CardiogenicDokumen4 halamanCardiovascular Changes in CardiogenicBaso AgusofyangBelum ada peringkat
- Overview of Cardiovascular System: An Introduction To Chapters 9 - 24 and Chapter 36 Guyton and Hall, 12 EditionDokumen10 halamanOverview of Cardiovascular System: An Introduction To Chapters 9 - 24 and Chapter 36 Guyton and Hall, 12 EditionbahahahahBelum ada peringkat
- Blood Pressure RegulationDokumen3 halamanBlood Pressure RegulationiMaibelle BelleBelum ada peringkat
- Chapter 7 Regulation of Arterial Blood Pressure and MicrocirculationDokumen20 halamanChapter 7 Regulation of Arterial Blood Pressure and Microcirculationaisyahasrii_Belum ada peringkat
- Cardiovascular Physiology: The Autonomic Nervous SystemDokumen8 halamanCardiovascular Physiology: The Autonomic Nervous SystemRidha Surya NugrahaBelum ada peringkat
- Preload and AfterloadDokumen4 halamanPreload and AfterloadNeranga SamaratungeBelum ada peringkat
- Sample ChapterDokumen14 halamanSample ChaptercpfredBelum ada peringkat
- Pulse PressureDokumen7 halamanPulse PressureChrisBelum ada peringkat
- Pinsky1997 Article TheHemodynamicConsequencesOfMeDokumen11 halamanPinsky1997 Article TheHemodynamicConsequencesOfMeAlvaro EstupiñánBelum ada peringkat
- Oscillometric Measurement of Systolic and Diastolic Blood Pressures Validated in A Physiologic Mathematical ModelDokumen22 halamanOscillometric Measurement of Systolic and Diastolic Blood Pressures Validated in A Physiologic Mathematical Modelejh261Belum ada peringkat
- Lilly Heart FailureDokumen29 halamanLilly Heart FailureQanita Izza KBelum ada peringkat
- Preload and AfterloadDokumen28 halamanPreload and Afterloadapi-19916399100% (1)
- Cardiovascular System Physiology, Lecture 2 (Cardiodynamics)Dokumen15 halamanCardiovascular System Physiology, Lecture 2 (Cardiodynamics)Sherwan R Shal100% (5)
- Mechanical Properties of The Heart IDokumen30 halamanMechanical Properties of The Heart ISeba W WolfBelum ada peringkat
- Chapter 66 NotesDokumen16 halamanChapter 66 NotesAlyssa Van VarkBelum ada peringkat
- CV A+P and CHFDokumen9 halamanCV A+P and CHFjazzdoc007Belum ada peringkat
- Stochastic Model For Blood Pressure AnalysisDokumen9 halamanStochastic Model For Blood Pressure AnalysisRakeshconclaveBelum ada peringkat
- Vasculopathy Emulation and Its Influence On The Cardiac Output Curve-Franyi Lorena DiazDokumen7 halamanVasculopathy Emulation and Its Influence On The Cardiac Output Curve-Franyi Lorena Diazfranyi lorenaBelum ada peringkat
- 5 - Cardiac Output - Ass. Prof. Dalia Abd El-Salam - 2020Dokumen6 halaman5 - Cardiac Output - Ass. Prof. Dalia Abd El-Salam - 2020Hossam BaniisBelum ada peringkat
- Fisiologia Del Retorno VenosoDokumen6 halamanFisiologia Del Retorno VenosoJorge FajardoBelum ada peringkat
- Lecture 14-Oct.23 Haemodynamics of Circulation, Circulatory Mechanics, ResistanceDokumen29 halamanLecture 14-Oct.23 Haemodynamics of Circulation, Circulatory Mechanics, ResistanceAparmitBelum ada peringkat
- Front Page: Effects of Systemic Vascular Resistance On The BodyDokumen1 halamanFront Page: Effects of Systemic Vascular Resistance On The BodyRodel Aguila SañoBelum ada peringkat
- Teknik Estimasi MAP: Itu Fungsi DariDokumen5 halamanTeknik Estimasi MAP: Itu Fungsi DariPrincess EzzlynnBelum ada peringkat
- Arterial Blood PressureDokumen7 halamanArterial Blood Pressuredhoha alawsiBelum ada peringkat
- Worksheet 5 APDokumen10 halamanWorksheet 5 APCDNBelum ada peringkat
- Frank-Starling Law of Cardiovascular DynamicsDokumen3 halamanFrank-Starling Law of Cardiovascular DynamicsSumirat NurcahyaniBelum ada peringkat
- CHF PathophysiologyDokumen4 halamanCHF PathophysiologyVirtudazo JessaBelum ada peringkat
- The Normal Physiology of The Heart: Vikkineshwaran Siva SubramaniamDokumen4 halamanThe Normal Physiology of The Heart: Vikkineshwaran Siva SubramaniamSivamala MalaBelum ada peringkat
- Ipi376947 PDFDokumen7 halamanIpi376947 PDFnurza yeyeniBelum ada peringkat
- Blood PressureDokumen8 halamanBlood PressureUmair RaoBelum ada peringkat
- Evaluation of Pumping Function of Heart: Stroke Volume Cardiac Output Ejection Fraction Cardiac IndexDokumen53 halamanEvaluation of Pumping Function of Heart: Stroke Volume Cardiac Output Ejection Fraction Cardiac Indexapi-19916399Belum ada peringkat
- II-A ShockDokumen5 halamanII-A ShockMuhammad075Belum ada peringkat
- Blood Pressure HomeostasisDokumen7 halamanBlood Pressure HomeostasisHassan Al SinanBelum ada peringkat
- (O. Nelson) Small Animal CardiologyDokumen208 halaman(O. Nelson) Small Animal CardiologyLoredana Gutu100% (1)
- 03C Modeling A Heart Pump PDFDokumen20 halaman03C Modeling A Heart Pump PDFMarlon Andres Navarro AlvarezBelum ada peringkat
- Heart FailureDokumen29 halamanHeart Failuremerin sunilBelum ada peringkat
- Blood Flow ECMDokumen31 halamanBlood Flow ECMirene :]Belum ada peringkat
- The Circulatory System Group 2Dokumen17 halamanThe Circulatory System Group 2sanique peterkinBelum ada peringkat
- Controlled HypotensionDokumen21 halamanControlled HypotensionChristian YonathanBelum ada peringkat
- Small Animal Cardiology PDFDokumen208 halamanSmall Animal Cardiology PDFRoberto AmpueroBelum ada peringkat
- Retorno Venoso y Presion de Llenado SistemicoDokumen11 halamanRetorno Venoso y Presion de Llenado SistemicoLenin BlancasBelum ada peringkat
- 12015exr PDFDokumen10 halaman12015exr PDFWahidur Rahman RafsanBelum ada peringkat
- High Blood Pressure: Safe alternatives without drugsDari EverandHigh Blood Pressure: Safe alternatives without drugsPenilaian: 5 dari 5 bintang5/5 (2)
- 1077 CMCNCHDokumen11 halaman1077 CMCNCHمحمد عقيليBelum ada peringkat
- Blood Flows, Pressure N ResistanceDokumen5 halamanBlood Flows, Pressure N Resistancemaxwell amponsahBelum ada peringkat
- 2 The Initial Assessment: Prioritizing Care Delivery: Cliff EvansDokumen24 halaman2 The Initial Assessment: Prioritizing Care Delivery: Cliff EvansJa YaBelum ada peringkat
- CNS Path Pharm KelnerDokumen9 halamanCNS Path Pharm Kelnerjazzdoc007Belum ada peringkat
- CV A+P and CHFDokumen9 halamanCV A+P and CHFjazzdoc007Belum ada peringkat
- Pathophysiology / Pharmacology NotesDokumen4 halamanPathophysiology / Pharmacology Notesjazzdoc007Belum ada peringkat
- CNS Path Pharm KelnerDokumen9 halamanCNS Path Pharm Kelnerjazzdoc007Belum ada peringkat
- AI and Sonographer Cardiac AssessmentDokumen9 halamanAI and Sonographer Cardiac AssessmentAndré Muniz de MouraBelum ada peringkat
- Anexa 1 - Configuratie Ingenuity 128 PDFDokumen18 halamanAnexa 1 - Configuratie Ingenuity 128 PDFAnghel Florin100% (2)
- 1 s2.0 S0735109722056030 MainDokumen15 halaman1 s2.0 S0735109722056030 MainAkhmad HidayatBelum ada peringkat
- UGEO H60 Reference Manual EDokumen183 halamanUGEO H60 Reference Manual EOsmel HernandezBelum ada peringkat
- ALFREDO 22010112130140 Lap - KTI Bab7Dokumen19 halamanALFREDO 22010112130140 Lap - KTI Bab7titikBelum ada peringkat
- HeartFailure PDFDokumen95 halamanHeartFailure PDFOktavianus PrayitnoBelum ada peringkat
- Cardiac SystemDokumen7 halamanCardiac Systemsccctutor100% (3)
- LOGIQ E11LOGIQ E20 R3 English Advanced Reference Manual (Domestic) - UM - 5919872-1EN - 1Dokumen203 halamanLOGIQ E11LOGIQ E20 R3 English Advanced Reference Manual (Domestic) - UM - 5919872-1EN - 1Hani Al-NassBelum ada peringkat
- Presentation2 BREAST IMAGING - LatestDokumen53 halamanPresentation2 BREAST IMAGING - LatestLadipo Temitope AyodejiBelum ada peringkat
- Indicator PVR ManuscriptDokumen20 halamanIndicator PVR ManuscriptshanizaBelum ada peringkat
- Dental Care For Patients With Heart Failure: An UpdateDokumen9 halamanDental Care For Patients With Heart Failure: An UpdateGustavo A OrtegonBelum ada peringkat
- Openhrt 2021 001732Dokumen9 halamanOpenhrt 2021 001732benypermadiBelum ada peringkat
- Narrative PathophysiologyDokumen18 halamanNarrative PathophysiologyNica Georgelle Maniego SamonteBelum ada peringkat
- Nurbn 2012Dokumen4 halamanNurbn 2012shrsmBelum ada peringkat
- Overview of Cardiovascular System: An Introduction To Chapters 9 - 24 and Chapter 36 Guyton and Hall, 12 EditionDokumen10 halamanOverview of Cardiovascular System: An Introduction To Chapters 9 - 24 and Chapter 36 Guyton and Hall, 12 EditionbahahahahBelum ada peringkat
- SonoAce R5 Reference Manual P PDFDokumen150 halamanSonoAce R5 Reference Manual P PDFNeto Infomab MedBelum ada peringkat
- Sample Article From JournalDokumen5 halamanSample Article From JournalnardiniyanBelum ada peringkat
- Acupuncture Heart FailureDokumen11 halamanAcupuncture Heart Failureabraham rumayaraBelum ada peringkat
- Ebook Egans Fundamentals of Respiratory Care 11Th Edition Kacmarek Test Bank Full Chapter PDFDokumen40 halamanEbook Egans Fundamentals of Respiratory Care 11Th Edition Kacmarek Test Bank Full Chapter PDFalexandercampbelldkcnzafgtw100% (11)
- Hot Topics in Tetralogy of FallotDokumen12 halamanHot Topics in Tetralogy of FallotqanitaBelum ada peringkat
- Alterations in Ventricular Pumping in Patients With Atrial Septal Defect at Rest, During Dobutamine Stress and After Defect ClosureDokumen10 halamanAlterations in Ventricular Pumping in Patients With Atrial Septal Defect at Rest, During Dobutamine Stress and After Defect ClosureYusrina Njoes SaragihBelum ada peringkat
- Low Ejection FractionDokumen6 halamanLow Ejection FractionSanjay kumarBelum ada peringkat
- ESC/EACTS vs. ACC/AHA Guidelines For The Management of Severe Aortic StenosisDokumen17 halamanESC/EACTS vs. ACC/AHA Guidelines For The Management of Severe Aortic StenosisMiguel PugaBelum ada peringkat
- 2articolo Base Sdi 2009Dokumen8 halaman2articolo Base Sdi 2009cesareBelum ada peringkat
- Spesifikasi Philips Incisive CT 128 PremiumDokumen2 halamanSpesifikasi Philips Incisive CT 128 PremiumvitapabloBelum ada peringkat
- Philips Ie33 Ultrahang ProspektusDokumen16 halamanPhilips Ie33 Ultrahang ProspektusNeimar R. LazarettiBelum ada peringkat
- Protocols and Methodologies in Basic Science and Clinical Cardiac MRI (PDFDrive)Dokumen445 halamanProtocols and Methodologies in Basic Science and Clinical Cardiac MRI (PDFDrive)Roberto DuniBelum ada peringkat
- Case StudyDokumen4 halamanCase StudyDaniel Angelo ArangoBelum ada peringkat
- Vivid S70N-English-brochure - JB80430XXDokumen18 halamanVivid S70N-English-brochure - JB80430XXArrief nurcahyo100% (1)
- Nejmra 2207410Dokumen15 halamanNejmra 2207410rindayusticia100% (1)