Answer Module 8A Acid and Base II
Diunggah oleh
Yen ZyHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Answer Module 8A Acid and Base II
Diunggah oleh
Yen ZyHak Cipta:
Format Tersedia
TIUSYEN IBNU SINA ANSWER SCHEME
Neutralisation
CHEMISTRY MODULE 8A ACID AND BASE II
1 100 cm3 of 0.2 mol dm-3 of potassium hydroxide aqueous, KOH reacts with 0.2 mol dm-3 of dilute hydrochloric acid, HCl. a) Write the chemical equation for the reaction. KOH + b) HCl KCl + H2O
Find the volume of acid used. 0.1 dm3 // 100 cm3
2 25 cm3 of 0.4 moldm-3 of potassium hydroxide aqueous, KOH reacts with 20 cm3 dilute sulphuric acid, H2SO4. (a ) (b ) Write the chemical equation for the reaction. 2 KOH + H2SO4 Find the molarity of acid used. 0.25 mol dm-3 K2SO4 + 2H2O
3 100 cm3 of 0.5 mol dm-3 of dilute sulphuric acid reacts with 0.2 mol dm-3 barium (II) hydroxide aqueous, Ba(OH)2. (a ) (b ) Write the chemical equation for the reaction. H2SO4 + Ba(OH)2 BaSO4 + 2 H20
Find the volume of barium (II) hydroxide aqueous used in this reaction. 250 cm3
4 30 cm3 of sodium hydroxide aqueous reacts with 15 cm3 of 0.1 moldm-3 of dilute sulphuric acid. (a ) Write the chemical equation for the reaction. H2SO4 (b ) + 2NaOH Na2 SO4 + 2 H20
Find the concentration of aqueous sodium hydroxide . 0.1 mol dm-3
TIUSYEN IBNU SINA ANSWER SCHEME
5
CHEMISTRY MODULE 8A ACID AND BASE II
Diagram 1 shows the set-up of apparatus for the neutralisation reaction between a strong acid and a strong alkali
Hydrochloric acid, 0.1 mol dm-3
25 cm3 sodium hydroxide solution + phenolphthalein DIAGRAM 1 25.0 cm3 of sodium hydroxide solution is poured into a conical flask. A few drops of phenolphthalein are added into the solution. The solution in the conical flask is titrated with 0.1 mol dm-3 hydrochloric acid .
(a)
Hydrochloric acid is a strong acid. What is meant by a strong acid? An acid that dissociate/ionise completely in water to produce high concentration of hydrogen ion
(b)
Suggest an apparatus that can be used to measure 25.0 cm3 of sodium hydroxide solution accurately. Burette//pipette State the colour change of the solution in the conical flask at the end point. Pink to colourless (i) Write a chemical equation for the above reaction. HCl + NaOH (ii) NaCl + H2O
(c) (d)
In this experiment, 20.0 cm3 hydrochloric acid is needed to neutralise 25.0 cm3 of
TIUSYEN IBNU SINA ANSWER SCHEME
sodium hydroxide solution.
CHEMISTRY MODULE 8A ACID AND BASE II
Calculate the molarity of the sodium hydroxide solution. 0.1 x Mb x 20 = 1 25 1
25 Mb = 2 Mb = 0.08 mol dm-3
(e)
(i)
The experiment is repeated with 0.1 mol dm-3 sulphuric acid to replace hydrochloric acid. Predict the volume of sulphuric acid needed to neutralise 25.0 cm3 sodium hydroxide solution. 10 cm3 Explain your answer in (e) (i). Sulphuric acid is a diprotic acid // hydrochloric acid is a monoprotic acid, so the number of hydrogen ion in sulphuric acid is twice compared to hydrochloric acid
(ii)
6 Malic acid is a weak acid which is found naturally in a wide variety of unripe fruits and in green apples. A student carried out the following experiment to determine the basicity of malic acid in some malic acid powder which was extracted from apple juice.
5.00 g of malic acid powder was dissolved in a little distilled water and was put into a conical flask.Three drops of phenolphthalein indicator were then added.
TIUSYEN IBNU SINA ANSWER SCHEME
CHEMISTRY MODULE 8A ACID AND BASE II
The content of the conical flask was titrated using standard sodium hydroxide solution. Complete neutralisation of malic acid required 37.30 cm3 of 2.00 mol dm-3 of sodium hydroxide solution.
a)
What is meant by weak acid? A weak acid is an acid that dissociates partially in water to produce hydrogen ion
b) c) d)
What is the taste of malic acid? sour What is the colour change at the end point? .colourless to pink Write the ionic equation for the neutralisation reaction. H+ + OHH2O
e)
(i)
Calculate the number of moles of malic acid in the sample. [Relative molecular mass of malic acid = 134.0] No of mol = 5 134 = 0.0373 mol
(ii)
Show by calculation that malic acid is a diprotic acid. 2 x 37.30 1000 = 0.0746 mol
TIUSYEN IBNU SINA ANSWER SCHEME
CHEMISTRY MODULE 8A ACID AND BASE II
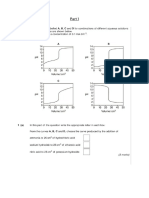
A student carried out two experiments to determine the concentration of HX acid solution. Diagram 7.1 shows the initial and final burette readings for both experiments. Experiment I Titration of HX acid solution of unknown concentration with 20 cm3 of sodium hydroxide solution 1.0 mol dm-3 using phenolftalein as indicator.
10
30
11
Initial burette reading
31
Final burette reading
Experiment II Titration of HX acid solution with different concentrations with 20 cm3 of sodium hydroxide solution 1.0 mol dm-3 using phenolphtalein as indicator.
10
20
11
21
Initial burette reading
Final burette reading Diagram 7.1
TIUSYEN IBNU SINA ANSWER SCHEME
CHEMISTRY MODULE 8A ACID AND BASE II
(a)
Construct a table to record the initial and final burette readings and the volumes of the acid used for both experiments. Experiment Initial reading/ cm3 Final reading/ cm3 Volume of HX/ cm3 I 10.45 30.45 20.00 II 10.45 20.45 10.00
(b)
State the colour change of phenolphtalein in the titration. Pink to colourless
(c)
The chemical equation for the reaction in the experiment is : HX + NaOH NaX + H2O
Calculate the concentration of HX acid solutions used in both experiments. Experiment I Concentration of acid = 1 x 20 / 20 Experiment II Concentration of acid = 1 x 20 / 10 (d) State the hypothesis in this experiment. The higher/ lower the concentration of HX acid , the lower/ higher the volume of acid needed to neutralize the sodium hydroxide solution (e) If HX acid is replaced with sulphuric acid of the same concentration, it is found that the volume of the sulphuric acid used in the titration is half of the volume of HX acid. Explain why. HX is monoprotic acid/ monobasic acid Sulphuric acid is diprotic/dibasic acid Number of hydrogen ion in 1 mol of sulphuric acid is double the amount compared to I mol of HX acid/ = 2.0 mol dm-3 = 1.0 mol dm-3
TIUSYEN IBNU SINA ANSWER SCHEME
CHEMISTRY MODULE 8A ACID AND BASE II
Anda mungkin juga menyukai
- CHE 123 HWK Back and Redox TitrationsDokumen3 halamanCHE 123 HWK Back and Redox TitrationsJuiloBelum ada peringkat
- S7 11012021 Acid Base Titrations WS With ANSWERSDokumen7 halamanS7 11012021 Acid Base Titrations WS With ANSWERSFatima Ahmed-VeriterBelum ada peringkat
- Some Basic Concepts of ChemistryDokumen12 halamanSome Basic Concepts of ChemistryNikhil BhattBelum ada peringkat
- Applications of Redox ReactionsDokumen50 halamanApplications of Redox ReactionsMlamuli MlarhBelum ada peringkat
- CHEM 1235 Volumetric QuizDokumen1 halamanCHEM 1235 Volumetric QuizJesseca Calaunan QuintoBelum ada peringkat
- PS1Dokumen1 halamanPS1Elah PalaganasBelum ada peringkat
- Anang 13Dokumen352 halamanAnang 13Jack JackBelum ada peringkat
- BT HPTDokumen31 halamanBT HPTLinh NguyenBelum ada peringkat
- Exp 1 Colloids SiapDokumen8 halamanExp 1 Colloids SiapFairuz Naim Z100% (1)
- Reclaiming Public Water: Achievements, Struggles and Visions From Around The WorldDokumen144 halamanReclaiming Public Water: Achievements, Struggles and Visions From Around The WorldNunoSacramentoBelum ada peringkat
- Back TitrationDokumen2 halamanBack TitrationjohnBelum ada peringkat
- Ranhill Watershed Years MIDF 151018 PDFDokumen16 halamanRanhill Watershed Years MIDF 151018 PDFchairunnisa cnaBelum ada peringkat
- Post-Laboratory Assignment. PROP 344Dokumen3 halamanPost-Laboratory Assignment. PROP 344bencleese100% (3)
- Chemistry ReportDokumen5 halamanChemistry ReportAngel Trisha Mae DelMundoBelum ada peringkat
- Argento Me TryDokumen5 halamanArgento Me TryGino GalanoBelum ada peringkat
- Lab 6 Using PH ElectrodeDokumen14 halamanLab 6 Using PH ElectrodeMina VoBelum ada peringkat
- Exp 2 Redox Inorganic ChemistryDokumen11 halamanExp 2 Redox Inorganic ChemistryAhmad Rawi100% (1)
- Gain Familiarity With Some of The Acid-Base, Oxidation-Reduction and Complexion Reaction of The Elements of The First Transition Series.Dokumen11 halamanGain Familiarity With Some of The Acid-Base, Oxidation-Reduction and Complexion Reaction of The Elements of The First Transition Series.FarahSyazwani100% (1)
- Sample Question 3 With AnswerDokumen18 halamanSample Question 3 With AnswerPyae Sone Kyaw100% (1)
- Acid Base TitrationDokumen57 halamanAcid Base TitrationRichard Obinna100% (1)
- Exp 3 Antacid TabletDokumen3 halamanExp 3 Antacid TabletMsShu9367% (3)
- CHM475 Exp10Dokumen4 halamanCHM475 Exp10Izzati HalidBelum ada peringkat
- Redox Titration ExptDokumen3 halamanRedox Titration ExptHetBelum ada peringkat
- Oxalate TitrationDokumen10 halamanOxalate Titrationlushu851648Belum ada peringkat
- Redox Electrochem H2 QuestionsDokumen7 halamanRedox Electrochem H2 QuestionskitoniumBelum ada peringkat
- Preparation of TetraamminecopperDokumen3 halamanPreparation of TetraamminecopperJana Zre2Belum ada peringkat
- UTAR Chem Lab 1 Full Report Exp12Dokumen7 halamanUTAR Chem Lab 1 Full Report Exp12Izykiel EdwardBelum ada peringkat
- Experiment 3 Redox Titration Percent Purity AnalysisDokumen5 halamanExperiment 3 Redox Titration Percent Purity AnalysisnanaBelum ada peringkat
- Experiment No 1 PDFDokumen3 halamanExperiment No 1 PDFVaid RahulBelum ada peringkat
- Inorganic Prac 2Dokumen3 halamanInorganic Prac 2Ray DyerBelum ada peringkat
- TitrationDokumen20 halamanTitrationrafiq84Belum ada peringkat
- Anal Chem 3 - Test 1-2016Dokumen4 halamanAnal Chem 3 - Test 1-2016Buhle BuhleBelum ada peringkat
- Experiment 1Dokumen4 halamanExperiment 1JasmeetSinghBelum ada peringkat
- Electrochemistry: Physical ChemistryDokumen32 halamanElectrochemistry: Physical ChemistryDavidson ChanBelum ada peringkat
- Lab Titration of VinegarDokumen5 halamanLab Titration of Vinegardesree07Belum ada peringkat
- Iron Lab ReportDokumen3 halamanIron Lab ReportaizatulsakuraBelum ada peringkat
- Title: K (Cu (C O) ) .2H ODokumen10 halamanTitle: K (Cu (C O) ) .2H ObabeBelum ada peringkat
- Instrumental Analytical Methods Experiment 8 - Conductometric Titration of Sulfuric Acid With Sodium BaseDokumen3 halamanInstrumental Analytical Methods Experiment 8 - Conductometric Titration of Sulfuric Acid With Sodium Baseapi-235187189Belum ada peringkat
- Experiment 4 CHM421Dokumen9 halamanExperiment 4 CHM421Abg Khairul Hannan Bin Abg AbdillahBelum ada peringkat
- Chemistry 02Dokumen6 halamanChemistry 02Towfiq Hossain TaskuBelum ada peringkat
- To Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Dokumen12 halamanTo Study The Kinetics of Persulphate-Iodide Ion Reaction by Initial Rate Method (Iodine Clock Reaction)Nishika GeraBelum ada peringkat
- Quantitative Determination of Phosphorus in Plant Food Using Household ChemicalsDokumen3 halamanQuantitative Determination of Phosphorus in Plant Food Using Household ChemicalsMaryBelum ada peringkat
- CHE485 - Lab Report On Determination ofDokumen25 halamanCHE485 - Lab Report On Determination ofAshton DykstraBelum ada peringkat
- Tritation Lab ReportDokumen8 halamanTritation Lab Reportapi-343706830Belum ada peringkat
- CH425Dokumen35 halamanCH425Vatra ReksaBelum ada peringkat
- Using KSP For The Dissolution of Borax in Water To Determine: G°, H° and S°Dokumen4 halamanUsing KSP For The Dissolution of Borax in Water To Determine: G°, H° and S°Valentin-AngeloUzunov100% (18)
- 2017F1110 Review Back Titration SlidesDokumen4 halaman2017F1110 Review Back Titration SlidesPeachBelum ada peringkat
- Acids and Bases PhETDokumen3 halamanAcids and Bases PhETChris GayleBelum ada peringkat
- Synthesis of Cobalt ComplexDokumen6 halamanSynthesis of Cobalt ComplexRenniel Pena100% (1)
- CopperDokumen3 halamanCopperdhungelsubhash8154Belum ada peringkat
- Analysis of BleachDokumen3 halamanAnalysis of BleachMatt VittingBelum ada peringkat
- Iodimetric Titration: Aim: PrincipleDokumen2 halamanIodimetric Titration: Aim: PrincipleHarsh ThakurBelum ada peringkat
- Linkage IsomersDokumen61 halamanLinkage IsomersMonica NC67% (3)
- Acid Dissociation ConstantDokumen4 halamanAcid Dissociation ConstantJair RangelBelum ada peringkat
- Experiment 10: Iodine Clock ReactionDokumen11 halamanExperiment 10: Iodine Clock ReactionJohn NdambukiBelum ada peringkat
- Buffers Complete Handout 2020 With Answer KeyDokumen14 halamanBuffers Complete Handout 2020 With Answer KeyRadhika RaniBelum ada peringkat
- Review KTT212Dokumen92 halamanReview KTT212Mohd HisyamBelum ada peringkat
- Chem 28.1 Problem Set Coplex TitrationsDokumen1 halamanChem 28.1 Problem Set Coplex TitrationsIda Anne Cacharel FuentespinaBelum ada peringkat
- Quantitative Determination of Copper Concentration in Aqueous Solution by Iodometric TitrationDokumen2 halamanQuantitative Determination of Copper Concentration in Aqueous Solution by Iodometric TitrationCaLee Macapagal100% (2)
- Titation and Limiting ReagentDokumen27 halamanTitation and Limiting Reagentngah lidwine100% (1)
- Answer Module 9A - Salt IDokumen2 halamanAnswer Module 9A - Salt IYen ZyBelum ada peringkat
- Answer Module 9A - Salt IDokumen2 halamanAnswer Module 9A - Salt IYen ZyBelum ada peringkat
- Answer Module 9A - Salt IDokumen2 halamanAnswer Module 9A - Salt IYen ZyBelum ada peringkat
- Answer Module 9A - Salt IDokumen2 halamanAnswer Module 9A - Salt IYen ZyBelum ada peringkat
- Answer Module 11A-Manufactured Substances in IndustryDokumen7 halamanAnswer Module 11A-Manufactured Substances in IndustryYen ZyBelum ada peringkat
- Answer Module 10A Salt1Dokumen6 halamanAnswer Module 10A Salt1Yen ZyBelum ada peringkat
- Answer Module 7A Acid and BasesDokumen5 halamanAnswer Module 7A Acid and BasesYen ZyBelum ada peringkat
- Answer Module 9A - Salt IDokumen2 halamanAnswer Module 9A - Salt IYen ZyBelum ada peringkat
- SemDokumen1 halamanSemjakelowBelum ada peringkat
- Materials and Design: Ehab A. El-Danaf, Magdy M. El-Rayes, Mahmoud S. SolimanDokumen6 halamanMaterials and Design: Ehab A. El-Danaf, Magdy M. El-Rayes, Mahmoud S. Solimankamal touilebBelum ada peringkat
- Cell Wall: Presented by M. Vijaya LakshmiDokumen9 halamanCell Wall: Presented by M. Vijaya LakshmiATCHUNALA SAIBelum ada peringkat
- CH 2 Chemical Bonding ICSE Solutions Class 10 ChemistryDokumen18 halamanCH 2 Chemical Bonding ICSE Solutions Class 10 ChemistrylionelkenethBelum ada peringkat
- Drug Education: September 9, 2017 Mati Davao OrientalDokumen119 halamanDrug Education: September 9, 2017 Mati Davao OrientalYem Binobo NantesBelum ada peringkat
- Structure Based Drug DesignDokumen91 halamanStructure Based Drug DesignMariamBelum ada peringkat
- ICSE Chemistry Board Paper19 PDFDokumen9 halamanICSE Chemistry Board Paper19 PDFPrajakta DigheBelum ada peringkat
- TM 10-4930-220-13PDokumen133 halamanTM 10-4930-220-13PAdvocateBelum ada peringkat
- Benzocaine Synthesis PDFDokumen2 halamanBenzocaine Synthesis PDFLive FlightsBelum ada peringkat
- Cleaning Validation MACO v2.1Dokumen3 halamanCleaning Validation MACO v2.1Syifa FatasyaaBelum ada peringkat
- Table 1: Patient's Response On The Effectiveness of The Aratiles Leaves TeaDokumen11 halamanTable 1: Patient's Response On The Effectiveness of The Aratiles Leaves TeaAlice Del Rosario CabanaBelum ada peringkat
- Test1 Goc & Poc Tough by S.K.sinha See Chemistry Animations atDokumen3 halamanTest1 Goc & Poc Tough by S.K.sinha See Chemistry Animations atmyiitchemistry100% (1)
- Gravimetric Analysis Laboratory ReportDokumen9 halamanGravimetric Analysis Laboratory ReportShawn RizalBelum ada peringkat
- Effect of Corrosion in StructuresDokumen32 halamanEffect of Corrosion in StructuresasvihariBelum ada peringkat
- Quiz BiochemistryDokumen100 halamanQuiz BiochemistryMedShare88% (25)
- 2014 Online Catlog - PDF MonDokumen50 halaman2014 Online Catlog - PDF Monjaag2000Belum ada peringkat
- Completions and WorkoverDokumen309 halamanCompletions and WorkoverFan Jack67% (3)
- Multiple Choice Questions (MCQ) Topic Quiz Biological MembranesDokumen22 halamanMultiple Choice Questions (MCQ) Topic Quiz Biological MembranesVeluz Kirt Peter GuiribaBelum ada peringkat
- SLR Strainer Data SheetDokumen7 halamanSLR Strainer Data SheetKailas NimbalkarBelum ada peringkat
- Alcohols (The Production Of)Dokumen15 halamanAlcohols (The Production Of)verity glenBelum ada peringkat
- Manual Overlay WeldingDokumen8 halamanManual Overlay Weldingcarlmac6183% (6)
- Raw Materials-IronDokumen22 halamanRaw Materials-IronAilson Silva AlvesBelum ada peringkat
- Spesifikasi Material Finishing Unit ApartemenDokumen34 halamanSpesifikasi Material Finishing Unit ApartemenArif GumelarBelum ada peringkat
- Presenters Post16 Tcm18-118246Dokumen18 halamanPresenters Post16 Tcm18-118246Kamariah IsmailBelum ada peringkat
- Work Instructions (W.I.)Dokumen18 halamanWork Instructions (W.I.)Shamsul Azhar MohdBelum ada peringkat
- 10 Scientist Contributed in ChemistryDokumen4 halaman10 Scientist Contributed in ChemistryJefferd PaetBelum ada peringkat
- Extraccion 4 PDFDokumen9 halamanExtraccion 4 PDFsergioenriquerozoperBelum ada peringkat
- Biomechanical Properties of A New Fiber-Reinforced CompositesDokumen10 halamanBiomechanical Properties of A New Fiber-Reinforced Compositesazam ahmedBelum ada peringkat
- HW 03 On IUPAC NamingDokumen1 halamanHW 03 On IUPAC NamingEMERALDARCANISTBelum ada peringkat
- 0423a ASKIN XFLAM Performance Panel Roofing-1Dokumen11 halaman0423a ASKIN XFLAM Performance Panel Roofing-1MacBelum ada peringkat