Group Report 1 Analytical Chemistry
Diunggah oleh
sssxxx2ndDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Group Report 1 Analytical Chemistry
Diunggah oleh
sssxxx2ndHak Cipta:
Format Tersedia
Group 2 Fransiskus Adithya Ivan Ardianto Puspita Anggreaini William Andreas
Group 2
Group Report 1 Analytical Chemistry
Electrochemistry and Potentiometry
Group Report PBL-1 Analytical Chemistry Group 1
Table of Contents
Problem Definition.............................................................................................................................3 Background Theory ............................................................................................................................3 Electrochemistry ................................................................................................................................3 Oxidation/ Reduction Concept ................................................................................................................. 3 Types of Electrochemical Cells .................................................................................................................. 3 Effect of Concentration on Electrode Potentials The Nernst Equation ................................................. 4 NiCd Battery .............................................................................................................................................. 4 Memory Effect .......................................................................................................................................... 5 Application of NiCd Battery and compared with other types of batteries ............................................... 5 Impact in environment ............................................................................................................................. 6 Potentiometry ...................................................................................................................................6 Direct Potentiometric technique .............................................................................................................. 6 Standard Addition technique .................................................................................................................... 7 Excess standard addition and Direct Potentiometric .............................................................................. 7 Trigger Problem Answers ...................................................................................................................7 Assignment I.............................................................................................................................................. 7 Assignment II........................................................................................................................................... 12 Assignment III.......................................................................................................................................... 14 References....................................................................................................................................... 16 Learning Scheme (Mindmap) of Electrochemistry and Potentiometry.................................................16
2|Page
Group Report PBL-1 Analytical Chemistry Group 1
Problem Definition
1. To understand the concept of batteries in correlation to the electrochemical concept. 2. To understand the concept of potentiometry to determine the concentration of heavy metals, such as copper.
Background Theory
Electrochemistry
Oxidation/ Reduction Concept
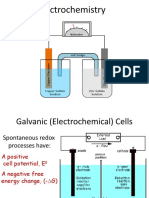
1. Oxidation Reduction reactions can be conducted in 2 ways : a. Direct contact between the oxidants and reductans. b. In a reaction in which the reactants do not come in direct contact with one another. 2. Example: Immersing a strip of copper (Cu) in a solution containing silver nitrate (AgNO3). Here, there is an oxidation and reduction reaction happens between the copper and the solution. Reduction (Silver Nitrate) CATHODE Oxidation (Cooper) ANODE Ag+ + e- Ag(s) Cu Cu2+ + 2e-
Oxidation and reactions has an unique characteristic of the transfer of electrons. Note that in the 2nd type of the oxidation reduction, a salt bridge isolates the reactants but maintain electrical contact between the CATHODE and ANODE cells. 3. The voltmeter measures the potential difference between the two metals at any instant, and there is a tendency of the potential decrease approaching 0 V as the reaction approaches the state of equilibrium. When zero voltage is reached, the concentrations of the 2 ions (in this case the Ag (I) and Cu (II) will have values that satisfy the equilibrium-constant expression for the net reduction/oxidation reaction 2 Ag+ + Cu(s) 2Ag(s) + Cu2+ At the equilibrium condition, there is no further flow of electrons will occurs.
Types of Electrochemical Cells
Electrochemical cells are either galvanic or electrolytic, and can also classified as reversible or irreversible. The table below describes the difference between the galvanic and electrolytic cells. Galvanic (Voltaic) Store electrical energy. Proceed spontaneusly. Electrolytic Need / consume electricity. Occurs at the reverse of the galvanic cell reactions. Cannot be conducted spontaneusly.
3|Page
Group Report PBL-1 Analytical Chemistry Group 1 A reversible cell is a electrochemical cell that can be reversed either in galvanic or electrolytic condition, while an irreversible cell, the cell cannot be reversed because it could cause an entirely different halfreaction to occur at both electrodes.
Effect of Concentration on Electrode Potentials The Nernst Equation
There is a quantitative relationship between concentration and the potential difference value of the electrodes. Consider the reversible half reaction: aA + bB + ... + ne- cC + dD + ... where the capital letters represent chemical formulas for the participating species (atoms, molecules, or ions), e- represents electrons, and the lower case italic letters indicates the number of moles of each species appearing in the half-reaction as it has been written. The electrode potential E for this process is described by the equation [ ] [ ] [ ] [ ] Where Eo = the standard electrode potential, which is a characteristics constant for each half-reaction R = the gas constant 8.314 J K-1 mol-1 T= temperature in kelvins n= number of moles of electrons that appear in the half-reaction for the electrode process as it has been written F= the faraday = 96,485 C (coulombs) Ln= the natural logarithm = 2.303 log Substituting numerical values for the constants, converting to base 10 logarithms, and specifying 25 oC for the temperature give [ ] [ ] [ ] [ ] Both equations above are named before Walther Hermann Nerst, a German physical chemist.
NiCd Battery
The nickel-cadmium battery (commonly abbreviated NiCd or NiCad ) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes . the components of NiCd battery are: Cathode Anode : Nickel (III) NiO(OH) : Cadmium
4|Page
Group Report PBL-1 Analytical Chemistry Group 1
A separator Electrolytes
: alkaline (KOH)
The reaction in electrodes when NiCd battery discharge:
(at anode)
(at cathode)
The reaction happen from left side to right side when discharge and rechargeable at the opposite.
Memory Effect
NiCd batteries may suffer from a " memory effect " if they are discharged and recharged to the same state of charge hundreds of times. The apparent symptom is that the battery "remembers" the point in its charge cycle where recharging began and during subsequent use suffers a sudden drop in voltage at that point, as if the battery had been discharged. if the device is unable to operate through this period of decreased voltage, it will be unable to get enough energy out of the battery, and for all practical purposes, the battery appears "dead" earlier than normal. The mechanism of memory effect is: the active material cadmium in NiCd Battery is small crystals which cover all the surface of NiCd cells. When memory effect happened, the crystals cover the active materials (Ni,Cd) and cause the drop voltage.
Application of NiCd Battery and compared with other types of batteries
NiCd battery use in many thing, for example the primary cell of NiCd battery usually use in electronic portable and toys. The main application of NiCd battery in cordless telephone and wireless, emergency lamps, and in the plane. Because of the low resistance, the NiCd battery has a high voltage current, and usually use in remote control, and camera, electric vehicles and standby power. Types of Batteries Type of Battery 1.Lead acid Battery Information Comparison with NiCd Battery
Have a higher density than NiCd. NiCd battery is smaller and Anode: Pb; Cathode: PbO2; lighter than lead acid battery Electrolyte : H2SO4. The main application in otomotive Irreversible chemical reaction in NiCd battery has smaller capacity alkaline battery. The voltage of and need high cost to produce.
2.Alcaline Battery
5|Page
Group Report PBL-1 Analytical Chemistry Group 1 alkaline battery was drop when NiCd battery last longer and charge drop. Many of them are keep the constant voltage when disposable battery. Anode: Zn; discharge. cathode: MnO2; electrolyte: Zink cloride 3.Lithium Ion Battery Rechargeable, and will broke NiCd battery when discharge in minimum (rechargeable) voltage. Anode: lithium; cathode and electrolyte: variation. The main application in phonecell, laptop, computer, and camera NiMH has bigger capacity and not toxic because the hydride adsorb the alloy in anode. Low cost to produce. The main applicationin hydride vechicle and prototype humanoid robot. last longer
4. Nickel Hydrida Battery (NiMH)
NiCd battery has low self discharge, 20%/ month (NiCd battery, 30%/month). NiCd not cause voltage decreases. Low resistance cause the high charge flow.
Impact in environment
All of the batteries have chemical materials which cause dangerous impact in environment. The most dangerous materials are heavy metals which carcinogenic, example: mercury and cadmium. It can cause substantial pollution when land filled or incinerated. Because of this, many countries now operate recycling programs to capture and reprocess old batteries. For the people, it can cause cancer, health problem, and died.
Potentiometry
Direct Potentiometric technique
This technique requires only an indicator of potential measurement of the electron when it is dipped in a solution containing an unknown concentration of an analyte and unknown. Indicator electrode is always considered as a cathode and reference electrode as the anode. For the direct potentiometry measurements, the cell potential can be expressed as a potential development by the indicator electrode, reference electrode, and potential functions. It depends on the potential difference measurement of electrodes in a solvent. Potential difference can be measured with pH meters/voltmeter. An electrodes is indicator electrode which use to give the response to the solvents.
6|Page
Group Report PBL-1 Analytical Chemistry Group 1 For calculations, the sign convention for direct potentiometry is the same as the convention mentioned in the general electrochemistry section for standard electrode potential. The indicator electrode is always treated as the right-hand electrode and the reference electrode as the left-hand electrode. The potential difference for the direct potentiometry follows this equation:
After a long derivation from the Nerstian form of the equation, and also considering the activity factor versus the concentration, the final equation that can be used: [ ] This equation effective for the cations. Meanwhile for the anions: [ ]
Standard Addition technique
This technique is commonly used in analytical instrumentation such as in atomic absorption spectroscopy and gas chromatography to find the value of the concentration of the substance (analyte) in a sample of unknown composition by comparison to samples of known concentration.
Excess standard addition and Direct Potentiometric
Calibration and measurement of samples done simultaneously so that the difference in ionic strength and temperature standards and the sample is not too significant. During the process, the electrodes remain immersed in the solution so that there is little change in junction potential solution. Measurement of slope very close to the concentration of the sample shows this method can yield more accurate results in the range of non-linear and can be used with electrodes old or older who was not linear range for the slope is stable.
Trigger Problem Answers
Assignment I
1. According to you why a particular black box on the plane should have its own backup power supply? What do you think are important issues to this topic? Answer: Backup power supply Because simply maintaining the operation of the flight data recorder would not be enough, all of the systems and sensors that require electrical power would also have to 7|Page
Group Report PBL-1 Analytical Chemistry Group 1 remain operative. Now for the cockpit voice recorder, we only need to power the recorder itself and the microphones in the cockpit. An independent power supply here might not be too difficult to implement. Because in the event of engine failure, larger aircraft are also equipped with emergency backup power sources like the auxiliary power generator and ram air turbine to continue operating the black boxes. In addition, we must consider to making a battery mandatory on solid-state recorders to provide an independent power supply in the event of a complete power failure aboard the plane. Black boxes are sometimes never found or too badly damaged to recover some or all of the data from a crash. To reduce the likelihood of damage or loss, some more recent designs are self-ejecting and use the energy of impact to separate themselves from the aircraft. So, we must provide the recorders with a backup battery to operate the devices for up to ten minutes if power is interrupted.
2. Because the paper will be presented in front of the jury who come from different disciplines, you are trying to make the summary of the concept and its relation to the electrochemical battery. What will you write? Answer: A Galvanic cell is an electrochemical cell that derives electrical energy from chemical reactions taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane. It is sometimes called a "Voltaic cell", after Alessandro Volta, inventor of the voltaic pile, the first electrical battery. In common usage, the word "battery" has come to include a single Galvanic cell, but a battery properly consists of multiple cells.
8|Page
Group Report PBL-1 Analytical Chemistry Group 1 A Galvanic cell consists of two half-cells. In its simplest form, each half-cell consists of a metal and a solution of a salt of the metal. The salt solution contains a cation of the metal and an anion to balance the charge on the cation. In essence the half-cell contains the metal in two oxidation states and the chemical reaction in the half-cell is an oxidation-reduction (redox) reaction, written symbolically in reduction direction as Mn+ (oxidized species) + n e M (reduced species)
In a galvanic cell one metal is able to reduce the cation of the other and, conversely, the other cation can oxidize the first metal. The two half-cells must be physically separated so that the solutions do not mix together. A salt bridge or porous plate is used to separate the two solutions yet keep the respective charges of the solutions from separating, which would stop the chemical reactions. The number of electrons transferred in both directions must be the same, so the two half-cells are combined to give the whole-cell electrochemical reaction. For two metals A and B: An+ + n e B
m+
A
m+
+me
B n B + m An+
mA+nB
When a metal in one half-cell is oxidized, anions must be transferred into that half-cell to balance the electrical charge of the cation produced. The anions are released from the other half-cell where a cation is reduced to the metallic state. Thus, the salt bridge or porous membrane serves both to keep the solutions apart and to allow the flow of anions in the direction opposite to the flow of electrons in the wire connecting the electrodes. The voltage of the battery is the sum of the voltages of the two half-cells. When a device such as an electric motor is attached to the electrodes, a current flows and redox reactions occur in both half-cells. This will continue until the concentration of the cations that are being reduced goes to zero.
3. From literature you read that type of batteries widely use in aircraft is the type of NiCd batteries. What shorts of thing are associated with this battery? It is true that NiCd batteries have memory effect?
Answer: NiCd Battery
9|Page
Group Report PBL-1 Analytical Chemistry Group 1
The nickel-cadmium battery (commonly abbreviated NiCd or NiCad ) is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes . The components of NiCd battery are: o o o o Cathode Anode A separator Electrolytes : Nickel (III) NiO(OH) : Cadmium : alkaline (KOH)
The reaction in electrodes when NiCd battery discharges:
(at anode)
(at cathode)
The reaction happen from left side to right side when discharge and rechargeable at the opposite. Memory Effect NiCd batteries may suffer from a " memory effect " if they are discharged and recharged to the same state of charge hundreds of times. The apparent symptom is that the battery "remembers" the point in its charge cycle where recharging began and during subsequent use suffers a sudden drop in voltage at that point, as if the battery had been discharged. if the device is unable to operate through this period of decreased voltage, it will be unable to get enough energy out of the battery, and for all practical purposes, the battery appears "dead" earlier than normal. The mechanism of memory effect is: the active material cadmium in NiCd Battery is small crystals which cover all the surface of NiCd cells. When memory effect happened, the crystals cover the active materials (Ni,Cd) and cause the drop voltage. 4. How do you think the development and application of NiCd battery compared to other battery? Do you know about zapping technique in improving battery performance? What do you think about environmental impact of disposable and rechargeable batteries?
Answer:
NiCd battery use in many thing, for example the primary cell of NiCd battery usually use in electronic portable and toys. The main application of NiCd battery in cordless telephone and wireless, emergency lamps, and in the plane. Because of the low resistance, the NiCd battery 10 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1 has a high voltage current, and usually use in remote control, and camera, electric vehicles and standby power. Types of Batteries Type of Battery 1.Lead acid Battery Information Comparison with NiCd Battery
Have a higher density than NiCd. NiCd battery is smaller Anode: Pb; Cathode: PbO2; and lighter than lead Electrolyte : H2SO4. The main acid battery application in automotive Irreversible chemical reaction in alkaline battery. The voltage of alkaline battery was drop when charge drop. Many of them are disposable battery. Anode: Zn; cathode: MnO2; electrolyte: Zink cloride NiCd battery has smaller capacity and need high cost to produce. NiCd battery last longer and keep the constant voltage when discharge.
2.Alcaline Battery
3.Lithium Ion Battery
Rechargeable, and will broke NiCd battery last longer when discharge in minimum (rechargeable) voltage. Anode: lithium; cathode and electrolyte: variation. The main application in phonecell, laptop, computer, and camera NiMH has bigger capacity and not toxic because the hydride adsorb the alloy in anode. Low cost to produce. The main applicationin hydride vechicle and prototype humanoid robot. NiCd battery has low self discharge, 20%/ month (NiCd battery, 30%/month). NiCd not cause voltage decreases. Low resistance cause the high charge flow.
4. Nickel Hydrida Battery (NiMH)
Impact in environment All of the batteries have chemical materials which cause dangerous impact in environment. The most dangerous materials are heavy metals which carcinogenic, example: mercury and cadmium. It can cause substantial pollution when land filled or incinerated. Because of this, many countries now operate recycling programs to capture and reprocess old batteries. For the people, it can cause cancer, health problem, and died.
11 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1 Disposable Battery Primer battery, ex: leclanche cell (1,5 V) Anode: Zn ; cathode: carbon tube ; electrolyte: MnO2 and NH4Cl Reaction : Zn(s)+2MnO2(s)+2NH4Cl(aq)ZnCl2+Mn2O3(s) +2NH3(aq)+H2O Used in telegraf, alarm, and low voltage instruments Rechargeable battery Secunder battery, ex: super iron battery. Cathode: Fe(VII), K2FeO4; anode: zink, electrolide: ammonium chloride and zink chloride. 50% capacity higher than alkaline battery. The new type battery develop in 2004
Assignment II
1. What reactions occur on each electrode in this electrochemical cell if the total reaction is written: ( Answer: In anode, the oxidation process occurs. The half reaction is: ( ) In cathode, the reduction process occurs. The half reaction is: ( ) 2. How to make electrolyte solution in order to obtain batteries with a voltage of 1.5 V at a temperature of 25oC? Answer: Using Nerst Equation: [ ] [ ] [ ] [ ] Standard Electrode Potential of: Mn (Reduction): +1,23 V Zn (Oxidation : +0,763 V Total Electrode Potential: +1,993 V Substituting the Potential and the Potential desired to the Nerst Equation: [ [ ] [ ] ] ) ( )
12 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1 [ [ [ [ [ [ [ [ [ ][ ] ][ ] [ ] ] [ ] ] ] [ ] ] ] [ ] ] ]
Corresponding to the unknown values of the concentration of [Mn3+],[Mn4+] or [Zn2+], the answer is given in ratio of both concentrations. 3. How do you explain the parameters of the battery capacity in amp-hour and battery voltage in V? How to estimate the life of batteries? Answer: Battery voltage is related to the potential difference between the two half reactions occured at the anode (oxidation reaction) and the cathode (reduction reaction). For example, the 1,5 Volt potential difference in common AA and AAA sized battery are derived from the half reaction of their cells, with the respective to their temperature and concentration (following the Nerst equation). The lifetime of batteries can be determined by the Peukerts Law:
Where : Qp : capacity when discharged at the rate of 1 amp. I : the current drawn from the battery. t : time the battery can sustain k : constant with a value about 1,3.
4. Why do you use graphite or carbon bar as electron flow collector on the cathode side nit only the MnO2 Answer: Carbon takes no part in the electrochemical reaction, but it collects the electrical current and reduce the resistance of the manganese dioxide mix.
13 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1
Assignment III
1. How do you determine the possibility of this river was polluted by copper from the industrial waste? Do you know what industries that have copper in their waste?
Answer:
Determination of copper content in the waste industry can be determined by the potential difference through which components such as reference electrode, indicator electrode, and salt bridges. These components can be interconnected to produce a potential difference. From this it can be determined the presence of copper content in the wastewater plant. Industry-industry wastes containing copper, among others. wire mills, metal coatings, pipes and others. 2. In the laboratory you have a pH meter/ volt meter, a standard saturated calomel electrode, and an indicator electrode for copper analysis. Because the committee will assess the project proposal, can you explain in the proposal the method of analysis to determine the content of copper ion in samples taken from the stream using existing equipment? The information is clear enough in terms of both instrumentation and theoretical basic principle of this analytical method.
Answer:
This is the technical set-up for the potentiometry method. The voltmeter is being set in series with the indicator electrode (in this case is the copper metal, because it is a copper concentration analysis) and the reference electrode (the standard saturated calomel electrode). Meanwhile the analyzed solution is poured at the glass beaker where both the indicator and reference cathode is being set. After the setup has been completed, we measure the voltage difference displayed at the voltmeter. The concentration of the analyzed solution can be determined by using the Nerst equation or the derivatives of the equation.
3. By using the direct potentiometric technique, you obtain data such as in figure. How do you determine the concentration of copper in the sample? Answer: From the table, we can collect some data such as: Slope: 0.0302V 14 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1 ECu-ISE: 0.2524V We also take the standard potential data of the reduction reaction of copper: We input the data into the nerst equation: [ [ [ [ ] ] ] ]
4. How do you explain the determination of copper concentration in the sample solution with standard addition technique? How do you explain the difference in direct potentiometric determination technique and standard addition technique?
Answer:
Step in addition standard technique: From the sample, determine the aliquot identical, Vx. adding a number of specific volume variation, Vs. its dilute each solution to a particular volume, Vt. Then calculate concentration on sample, Cu, with equation:
Cu=
Cu= concentration in unknown sample Cs= concentration in standard sample Vs= standard volume Vu= Sample volume E1= electro potential in pure solvent E2= electrode potential in addition m= electrode slope Direct Potentiometric technique This technique requires only an indicator of potential measurement of the electron when it is dipped in a solution containing an unknown concentration of an analyte and unknown. Indicator electrode is always considered as a cathode and reference electrode as the anode. For the direct potentiometric measurements, the cell potential can be expressed as a potential development by the indicator electrode, reference electrode, and potential functions. It depends on the potential difference measurement of electrodes in a solvent. Potential difference can be measured with pH meters/voltmeter. An electrodes is indicator electrode which use to give the response to the solvents. Standard Addition technique 15 | P a g e
Group Report PBL-1 Analytical Chemistry Group 1 This technique is commonly used in analytical instrumentation such as in atomic absorption spectroscopy and gas chromatography to find the value of the concentration of the substance (analyte) in a sample of unknown composition by comparison to samples of known concentration. Excess standard addition and Direct Potentiometric Calibration and measurement of samples done simultaneously so that the difference in ionic strength and temperature standards and the sample is not too significant. During the process, the electrodes remain immersed in the solution so that there is little change in junction potential solution. Measurement of slope very close to the concentration of the sample shows this method can yield more accurate results in the range of non-linear and can be used with electrodes old or older who was not linear range for the slope is stable.
References
1. Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch, Fundamentals of Analytical Chemistry 8th Edition. Saunders College Publishing, New York,2002. 2. http://en.wikipedia.org/wiki/Battery 3. http://en.wikipedia.org/wiki/potentiometry 4. http://en.wikipedia.org/wiki/Ni-Cd
16 | P a g e
Anda mungkin juga menyukai
- Che 1Dokumen31 halamanChe 1dineshsilambam2305Belum ada peringkat
- Chemistry For Engineers Laboratory: CHEM 114Dokumen8 halamanChemistry For Engineers Laboratory: CHEM 114Ivyy Joyce BuanBelum ada peringkat
- Electrochemical Cells R - Virtual LabDokumen3 halamanElectrochemical Cells R - Virtual LabJosua VivasBelum ada peringkat
- Batteries & Fuel Cells Dr. Siju N. AntonyDokumen31 halamanBatteries & Fuel Cells Dr. Siju N. AntonysijunantonyBelum ada peringkat
- Need For Alternate Energy SourcesDokumen17 halamanNeed For Alternate Energy SourcesshruniviBelum ada peringkat
- Electrochemistry: Assoc. Prof. Jacqui Lou Valenzuela, RCH Chemistry Department Cas, WitDokumen53 halamanElectrochemistry: Assoc. Prof. Jacqui Lou Valenzuela, RCH Chemistry Department Cas, WitJacquiBelum ada peringkat
- General Chemistry 2 Quarter 4: Week 7 - Module 7 Standard Cell Potential, Electrochemical Cells and BatteriesDokumen21 halamanGeneral Chemistry 2 Quarter 4: Week 7 - Module 7 Standard Cell Potential, Electrochemical Cells and BatteriesCamille Joves EncarnacionBelum ada peringkat
- Chapter 18 ElectrochemistryDokumen17 halamanChapter 18 ElectrochemistryNefliBelum ada peringkat
- Batteries: Types of Batteries Primary Batteries Secondary Batteries Fuel Cells / Flow BatteriesDokumen24 halamanBatteries: Types of Batteries Primary Batteries Secondary Batteries Fuel Cells / Flow BatteriesMahek50% (2)
- Battery TechnologyDokumen60 halamanBattery TechnologyKasinathan MuniandiBelum ada peringkat
- Electrochemical DevicesDokumen91 halamanElectrochemical DevicesSujalBelum ada peringkat
- Chem101 Ho4Dokumen4 halamanChem101 Ho4cyrusryan21Belum ada peringkat
- BatteryDokumen11 halamanBatteryHarsha VardhanBelum ada peringkat
- Batteries and Fuel CellsDokumen31 halamanBatteries and Fuel CellsTomesh SahuBelum ada peringkat
- Chapter6-Electrochemistry (Part 2)Dokumen27 halamanChapter6-Electrochemistry (Part 2)Uswatun KhasanahBelum ada peringkat
- Experiment 8 PhyChem IIDokumen5 halamanExperiment 8 PhyChem IIティン ヨロベ100% (1)
- Electrochemistry Chemistry and ElectricityDokumen54 halamanElectrochemistry Chemistry and ElectricityMaria OzaoBelum ada peringkat
- B.Tech First Year: Course Name: Engineering ChemistryDokumen29 halamanB.Tech First Year: Course Name: Engineering ChemistryHemant Singh JadounBelum ada peringkat
- Chem 131 Lesson 8Dokumen5 halamanChem 131 Lesson 8Denampo Ivan MikhaelBelum ada peringkat
- Chapter One: Introduction of ElectrochemistryDokumen29 halamanChapter One: Introduction of ElectrochemistryBayan O. Abu SaadaBelum ada peringkat
- Battery PDFDokumen7 halamanBattery PDFCHARITHABelum ada peringkat
- CH 3 14Dokumen135 halamanCH 3 14active learning educationBelum ada peringkat
- Lecture 4 - Electro Chem PDFDokumen49 halamanLecture 4 - Electro Chem PDFHedric VillenaBelum ada peringkat
- BatteriesDokumen22 halamanBatterieshariarun0705Belum ada peringkat
- Energy ResourcesDokumen6 halamanEnergy ResourcesAm AsdfghjklBelum ada peringkat
- Electrochemical Energy Systems: Batteries and Fuel CellsDokumen42 halamanElectrochemical Energy Systems: Batteries and Fuel Cellsshubhika guptaBelum ada peringkat
- Potentiometry - Without Voice Narration PDFDokumen35 halamanPotentiometry - Without Voice Narration PDFKalyavalla SathyasaiBelum ada peringkat
- M1 Battery TechnologyDokumen13 halamanM1 Battery TechnologyMalvika RkBelum ada peringkat
- ElectrochemistryDokumen27 halamanElectrochemistry22cs103Belum ada peringkat
- Chem 17Dokumen9 halamanChem 17Adi SoBelum ada peringkat
- Nickel Cadmium 1-WPS OfficeDokumen3 halamanNickel Cadmium 1-WPS OfficeWibi SonoBelum ada peringkat
- Experiment 14 - ElectrochemistryDokumen10 halamanExperiment 14 - ElectrochemistryArifmyBelum ada peringkat
- Department of Chemical EngineeringDokumen12 halamanDepartment of Chemical EngineeringSheikh AliBelum ada peringkat
- Battery Knowledge - : Contact UsDokumen4 halamanBattery Knowledge - : Contact UsSiva MurugesanBelum ada peringkat
- Chemistry BeginingDokumen5 halamanChemistry Beginingarchita1072006Belum ada peringkat
- Chapter6-Electrochemistry (Part 2)Dokumen27 halamanChapter6-Electrochemistry (Part 2)BagusprPrasetyoBelum ada peringkat
- Tutorial Sheet7Dokumen5 halamanTutorial Sheet7Lê Anh QuangBelum ada peringkat
- 0005unit V NotesDokumen33 halaman0005unit V Noteskishan kumarBelum ada peringkat
- Relating Values of Cell Potential: For General Chemistry 2/grade 12-STEM Quarter 4/week 8.b-cDokumen11 halamanRelating Values of Cell Potential: For General Chemistry 2/grade 12-STEM Quarter 4/week 8.b-cAllona Jane BrionesBelum ada peringkat
- Aircraft Electrics - Aircraft BatteriesDokumen17 halamanAircraft Electrics - Aircraft BatteriesErica Zoe BantogBelum ada peringkat
- RSC - ElectrochemistryDokumen98 halamanRSC - ElectrochemistrymokilpoBelum ada peringkat
- TSSM Topic 5Dokumen18 halamanTSSM Topic 5sudotesterBelum ada peringkat
- Lab Activity 7 ElectrochemistryDokumen8 halamanLab Activity 7 Electrochemistryjhunjhun zambranoBelum ada peringkat
- Chapter 3Dokumen30 halamanChapter 3Umesh ChandraBelum ada peringkat
- Batteries Chemistry ProjectDokumen12 halamanBatteries Chemistry ProjectAbhishek yadav67% (12)
- Potential of A Galvanic CellDokumen6 halamanPotential of A Galvanic CellRalph Andrew EsperonBelum ada peringkat
- Unit 12 ElectrochemistryDokumen22 halamanUnit 12 Electrochemistrycream oBelum ada peringkat
- Lesson 1 FunctionsDokumen7 halamanLesson 1 FunctionsWaien G. WatamamaBelum ada peringkat
- Voltaic Minicell LabDokumen6 halamanVoltaic Minicell LabIvy LongBelum ada peringkat
- ElectroDokumen13 halamanElectrodulalsushant3Belum ada peringkat
- Chapter Five Introduction To Electroanalytical ChemistryDokumen16 halamanChapter Five Introduction To Electroanalytical ChemistryZekarias LibenaBelum ada peringkat
- Class 12 Chemistry Revision Notes ElectrochemistryDokumen25 halamanClass 12 Chemistry Revision Notes ElectrochemistrySariska MehraBelum ada peringkat
- Step 1: Break Reaction Into Half-Reactions by IonsDokumen3 halamanStep 1: Break Reaction Into Half-Reactions by IonsjoenelBelum ada peringkat
- 5 - Electrochemistry PDFDokumen15 halaman5 - Electrochemistry PDFthinkiit100% (1)
- Electrochemical Cells Revised 12/8/14Dokumen7 halamanElectrochemical Cells Revised 12/8/14SamarpitBelum ada peringkat
- Electrochemistry - Galvanic Cell NewDokumen51 halamanElectrochemistry - Galvanic Cell NewPink WandererBelum ada peringkat
- Electro ChemistryDokumen2 halamanElectro Chemistryradhikanaveen60Belum ada peringkat
- The Stopping and Ranges of Ions in Matter: Handbook of Stopping Cross-Sections for Energetic Ions in All ElementsDari EverandThe Stopping and Ranges of Ions in Matter: Handbook of Stopping Cross-Sections for Energetic Ions in All ElementsBelum ada peringkat
- Solid-State Circuits: Electrical Engineering DivisonDari EverandSolid-State Circuits: Electrical Engineering DivisonPenilaian: 4.5 dari 5 bintang4.5/5 (4)
- Grove B4, B5 and B7 Side-Entry Ball ValvesDokumen32 halamanGrove B4, B5 and B7 Side-Entry Ball Valvesrobert rivasBelum ada peringkat
- Process Description For Effluent Treatment PlantDokumen2 halamanProcess Description For Effluent Treatment Plantyaxita talati50% (2)
- Biohall Germany Catalouge-2021-22Dokumen154 halamanBiohall Germany Catalouge-2021-22TRUCAL NABLBelum ada peringkat
- Spark TestingDokumen23 halamanSpark TestingJad MacintoshBelum ada peringkat
- Exergoeconomic Analysis of A Combined Heat and Power (CHP) SystemDokumen17 halamanExergoeconomic Analysis of A Combined Heat and Power (CHP) Systemjhugo_mirandaBelum ada peringkat
- Complex Systems in Finance and EconometricsDokumen19 halamanComplex Systems in Finance and EconometricsJuliana TessariBelum ada peringkat
- Polymer Repair PDFDokumen3 halamanPolymer Repair PDFamirouche15Belum ada peringkat
- ChemicalsDokumen72 halamanChemicalsMrudulaBelum ada peringkat
- Gasha International School Homework PolicyDokumen12 halamanGasha International School Homework PolicyOmar KhidhirBelum ada peringkat
- Solef Hylar PVDFDokumen60 halamanSolef Hylar PVDFribeiro30Belum ada peringkat
- Ce 8381 - Question PaperDokumen2 halamanCe 8381 - Question PaperculvertsBelum ada peringkat
- Synthesis and Kinetic Study of Co (Salen) (Revision)Dokumen3 halamanSynthesis and Kinetic Study of Co (Salen) (Revision)Daniel Rodman50% (2)
- New Atlanta Specifications For HdpeDokumen14 halamanNew Atlanta Specifications For HdpeMarvin MartinezBelum ada peringkat
- DehydrationDokumen13 halamanDehydrationSaa D ShamimBelum ada peringkat
- Calculation of Liquid Heat Capacity of Petroleum Distillate FuelsDokumen3 halamanCalculation of Liquid Heat Capacity of Petroleum Distillate Fuelsdennise8Belum ada peringkat
- Chelidonium Majus PDFDokumen40 halamanChelidonium Majus PDFbhaskarsgBelum ada peringkat
- Flowrate Calculation For A Draining TankDokumen2 halamanFlowrate Calculation For A Draining TankAnonymous bHh1L1Belum ada peringkat
- EmileorConcerningEducation 10106815Dokumen165 halamanEmileorConcerningEducation 10106815Túlio Coelho SampaioBelum ada peringkat
- Shell MFO 500 (Non ISO Grade) : Test Property Unit MethodDokumen1 halamanShell MFO 500 (Non ISO Grade) : Test Property Unit MethodVilius BukysBelum ada peringkat
- Solvent Sbps 1425 - HPCLDokumen2 halamanSolvent Sbps 1425 - HPCLBharat ChatrathBelum ada peringkat
- Environmental Chemistry and Microbiology - Unit 3 - Week 1Dokumen6 halamanEnvironmental Chemistry and Microbiology - Unit 3 - Week 1Abhijit NathBelum ada peringkat
- Question Paper CodeDokumen4 halamanQuestion Paper CodeBalaji ArunBelum ada peringkat
- Solutions NumericalsDokumen3 halamanSolutions Numericalsdevesh saiBelum ada peringkat
- ks3 Acids and Alkalis Whats Your Idea PowerpointDokumen11 halamanks3 Acids and Alkalis Whats Your Idea PowerpointManha abdellahBelum ada peringkat
- A Simplified Method For The Cultivation of Extreme Anaerobic Archaea Based SULFIDE 2000 !!!!Dokumen6 halamanA Simplified Method For The Cultivation of Extreme Anaerobic Archaea Based SULFIDE 2000 !!!!Vera Brok-VolchanskayaBelum ada peringkat
- Is 3025 - 31 - 1 - 2022Dokumen16 halamanIs 3025 - 31 - 1 - 2022Ruby MalhotraBelum ada peringkat
- Notes On MRI - FinalDokumen16 halamanNotes On MRI - FinalAnju GuptaBelum ada peringkat
- Biology O Level Notes PDFDokumen36 halamanBiology O Level Notes PDFGB Hacker100% (1)
- Remediation Project 1Dokumen26 halamanRemediation Project 1api-508660724Belum ada peringkat
- ISSN:2157-7048: Executive EditorsDokumen10 halamanISSN:2157-7048: Executive EditorsElaziouti AbdelkaderBelum ada peringkat