Experiment 2 Chromatography
Diunggah oleh
Chacha MercadoDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Experiment 2 Chromatography
Diunggah oleh
Chacha MercadoHak Cipta:
Format Tersedia
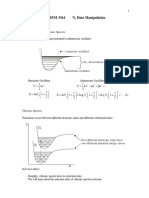
Experiment 2: Separation of Amino Acids by Paper Chromatography Chingcuangco, Ma. Renelyn D. Galan, Charize Mae U. Group 2, Chem 31.
1; AB2; Ms. Denise Arganda April 29, 2009 Abstract In any chemical or bioprocessing industry, the need to separate and purify a product from a complex mixture is a necessary and important step in the production line. Chromatography is a very special separation process; it can separate complex mixtures with great precision. Even very similar components, such as proteins that may only vary by a single amino acid, can be separated with chromatography. It is also used to separate and identify all sorts of substances in police work; drugs from narcotics to aspirin can be identified in urine and blood samples. It does not only provide methods of separation, chromatographic techniques can also provide methods of analysis. This experiment was done to demonstrate the separation of amino acids by paper chromatography and use chromatography to identify unknown amino acids. Definition of Terms: Chromatography a technique used to separate mixtures into components Paper Chromatography one of the methods of chromatography by using a paper like filter paper or any special paper Mobile Phase gas or liquid that carries the components Stationary Phase part of the system that does not move with the sample I. Introduction Chromatography is a powerful method to separate mixtures of substances into their respective substances. All forms of chromatography have a mobile phase, which can be a liquid or gas, and a stationary phase which can be a solid or liquid supported on a solid. As the sample is introduced into the mobile phase, the mobile phase flows through the stationary phase and caries the mixtures components with it. Different components travel at different rates due to various factors. Chromatographic procedures can be adsorption chromatography and partition chromatography. In adsorption chromatography, there is an equilibrium between the mobile and stationary phase which accounts for the separation of the components. In partition chromatography, the components are distributed between the mobile phase and the stationary liquid. II. Methodology 12 mL of butanol, 3 mL glacial acetic acid and 5 mL distilled water were mixed in a bottle without wetting the sides with the solvent and it was covered. A 19 cm x 14 cm Whatman No. 1 chromatography paper was laid on a clean sheet of bond paper and a very light pencil line was drawn across its length 1 cm from the bottom. Starting 2 cm from the left edge, light pencil cross marks were drawn on the line 3 cm apart. With a capillary tube, amino acid solution was applied four times at the center of the cross marks. The spots were dried first before applying the succeeding spots. The spots were labeled with pencil marks to identify the amino acids and the unknown. The paper was rolled into a cylinder and the edges were stapled 1 mm apart. It was then put inside the bottle containing the developing solvent with the sample edge down. The line containing the amino acid solution spots is not immersed in the solution. The bottle was closed tightly and was not moved while the solvent is rising. The process was stopped when the solvent front is only about 1 cm from the top edge of the paper. The paper was removed and was dried. The staples were removed and the paper was laid down on a clean bond paper with its front side down. Ninhydrin solution was brushed lightly and evenly on the paper along the direction of solvent flow under the dryer. The edges of the resulting violet spots were traced and the center with the darkest spot was marked. The Rf values of the amino acids were calculated and the unknown was identified. III. Results and Discussions The distances of the solute and solvent were measured and were taken as follows: Amino Acid Standards Glycine Distance Traveled by the amino acid (cm) 20 Distance Traveled by the Solvent (cm) 116 Page 1 of 3
Experiment 2: Separation of Amino Acids by Paper Chromatography
Lysine Leucine Tyrosine Unknown
102 70 49 41
116 116 116 116
porosity, direction of the papers fibers, and molecular weight of solute (mobile phase). 4. Give reasons for the following procedures. a. The diameter of the amino acid spots should be about 1mm only. The diameter of the amino acid spots should be about 1 mm only because if the spot is big enough, the solute might travel more in a horizontal manner than vertically and might come in contact with those amino acids on its side. b. The solvent mixture should be allowed to saturate the chromatography chamber. The chromatography chamber must be kept saturated so that the solvent will not evaporate while it rises up the filter paper. c. The chromatography paper should not be touched with bare hands. Sweat from the skin will contaminate the chemicals present and skins oils will also appear on the chromatography paper which will give inaccuracy with the results. 5. A mixture of amino acids was separated into its component by two-dimensional chromatography using solvents S1 and S2. The data obtained are given below: Distance traveled by solvent fronts: S1 (Butanol, acetic acid, water mixture) =11.5cm S2 (Phenol, water mixture) = 12 cm Distance traveled by the amino acid standard in S1 and S2: Amino Acid S1 (cm) S2 (cm) A 6.1 5.8 B 8.9 2.1 C 6.0 1.0 D 9.0 4.5 Distance traveled by the Amino Acid standard in S1 and S2: Amino Acid S1 (cm) S2 (cm) Ala 3.7 6.5 Phe 9.14 4.9 Lys 6.15 1.3 Leu 2.0 9.6 Glu 2.3 7.5 His 9.0 2.2 Trp 5.9 6.0 Draw clearly the two-dimensional chromatogram and indicate the directions of solvent flow. Identify amino acids A, B, C and D. Page 2 of 3
Based on the measurements obtained, we can get the Rf values of each amino acid by this formula: Rf = distance traveled by the amino acid distance traveled by the solvent Rf or Retention factor is the ratio between the distance traveled by the solute and the distance traveled by the solvent. This value is between 0 and 1 and has no unit. This is used to identify the unknown by comparing its Rf value to a Rf value of known compounds. Glycine = 0.17/116 = 0.17 Lycine = 102/116 = 0.88 Leucine = 70/116 = 0.60 Tyrosine = 49/116 = 0.42 Unknown = 41/116 = 0.35 Since the Rf value of the unknown amino acid, which is 0.35, is closest to the Rf value of Tyrosine, which is 0.42, we can identify this unknown as Tyrosine. IV. Guide Questions 1. Identify the stationary and mobile phase in paper chromatography. Stationary phase water Mobile phase Butanol 2. Explain briefly the differences in Rf values of the amino acid component of your mixture. The different polarity of the amino acids contribute to their differences in Rf values. Compounds with larger Rf have lesser polarity because it interacts less strongly with the polar adsorbent. 3. What are the factors that could affect the Rf value of a solute. Factors that could affect the Rf values are the solvent system polarity, adsorbent/paper
Experiment 2: Separation of Amino Acids by Paper Chromatography
A compound of low polarity will have larger R f value because it interacts less strongly with the polar adsorbent which is the filter paper. Different substances (amino acids) have different Rf values. Knowing the Rf values of the 4 basic amino acids, you can compare and know whats the identity of the unknown amino acid.
VI. References Carrier, Rebecca et al (1994). Intro to Chromatography.http://www.rpi.edu/dept/chemeng/Biotech-Environ/CHROMO/chromintro.html
6. Discuss briefly the basic principles of the following chromatographic techniques. a. Thin-Layer Chromatography It involves a stationary phase of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat, inert substrate. It has faster runs, better separations and the choice between different adsorbents compared to paper. b. Column Chromatography It consists of a column of particular material such as silica or alumina that has a solvent passed through it at atmospheric or low pressure. c. Gas Chromatography It involves a solid stationary phase and a mobile gas, most often Helium. The stationary phase adheres to the inside of a capillary column or a packed column. d. High Performance Chromatography It is a separational technique in which the mobile phase is a liquid. It utilizes very small packing particles, which is the stationary phase, and a relatively high pressure. e. Reversed Phase Chromatography It is a procedure used in liquid chromatography in which the mobile phase is significantly more polar than the stationary phase. V. Conclusion Difference in affinities of components of a mixture to the stationary and mobile phase determines the rate at which they travel on the filter paper. The larger the Rf value of a compound, the larger the distance it travels on the chromatogram.
I hereby certify that I have given substantial contribution to this report. Chingcuangco, Ma. Renelyn D. Galan, Charize Mae U.
Experiment 2: Separation of Amino Acids by Paper Chromatography
Page 3 of 3
Anda mungkin juga menyukai
- Separation and Identification of Amino Acids by Paper ChromatographyDokumen4 halamanSeparation and Identification of Amino Acids by Paper Chromatographyroxannediana86% (14)
- Biochemistry QuizDokumen28 halamanBiochemistry Quizsanjviews100% (3)
- Separation of Amino Acids by Paper ChromatographyDokumen38 halamanSeparation of Amino Acids by Paper ChromatographyGenny Marantan83% (6)
- TechReport Preparation of Buffer Solution L02-T1Dokumen45 halamanTechReport Preparation of Buffer Solution L02-T1Farihah Eyfa100% (1)
- Potentiometric TitrationDokumen9 halamanPotentiometric Titrationiah_guevarraBelum ada peringkat
- Extraction of Total Lipids From Chicken Egg Yolk and Column Chromatography of LipidsDokumen6 halamanExtraction of Total Lipids From Chicken Egg Yolk and Column Chromatography of LipidsJea CansinoBelum ada peringkat
- TLC Lipids Lab ReportDokumen12 halamanTLC Lipids Lab Reportvanessa olga100% (1)
- Experiment 9 Separation of Amino Acid Mixture by PDokumen6 halamanExperiment 9 Separation of Amino Acid Mixture by PNur AsyikinBelum ada peringkat
- Identification of Amino Acids-Paper ChromatographyDokumen9 halamanIdentification of Amino Acids-Paper ChromatographySharanya Srinivasan50% (2)
- Acid-Base Titrations 2Dokumen27 halamanAcid-Base Titrations 2John Henrick G. Uy100% (2)
- Problem of Spectroscopy: Teacher: Nguyen Thien ThaoDokumen49 halamanProblem of Spectroscopy: Teacher: Nguyen Thien Thaogianghha50% (2)
- Waters LC-MS PrimerDokumen44 halamanWaters LC-MS PrimerKiran ChokshiBelum ada peringkat
- Formal Report On Thin Layer ChromatographyDokumen2 halamanFormal Report On Thin Layer ChromatographyAthena OcampoBelum ada peringkat
- Monsanto Experiment 5 Amino AcidsDokumen6 halamanMonsanto Experiment 5 Amino AcidsRhey Christian MonsantoBelum ada peringkat
- Inorganic ManualDokumen49 halamanInorganic ManualAbrhsh100% (1)
- Fast Liquid Chromatography Mass Spectrometry Methods in Food and Environmental AnalysisDokumen625 halamanFast Liquid Chromatography Mass Spectrometry Methods in Food and Environmental AnalysisIvana NikolicBelum ada peringkat
- Volumetric AnalysisDokumen29 halamanVolumetric AnalysisReyia ApanteBelum ada peringkat
- LC MsDokumen24 halamanLC MsVshn VardhnBelum ada peringkat
- Thin Layer ChromatographyDokumen8 halamanThin Layer ChromatographyIsabel RinconBelum ada peringkat
- X-Ray Photoelectron Spectroscopy (XPS)Dokumen19 halamanX-Ray Photoelectron Spectroscopy (XPS)caonguyenbanso100% (1)
- Mass SpectrometryDokumen52 halamanMass Spectrometrybbhavya50% (2)
- Amino Acids by Paper ChromatographyDokumen3 halamanAmino Acids by Paper ChromatographynaomiBelum ada peringkat
- What Is Paper Chromatography? Principle and Procedure: Nature of The PaperDokumen8 halamanWhat Is Paper Chromatography? Principle and Procedure: Nature of The PaperWiz Micheal SmithBelum ada peringkat
- Lab Report 4 ChromatographyDokumen6 halamanLab Report 4 ChromatographyMyeeka Hammond100% (1)
- Experiment 4Dokumen9 halamanExperiment 4Peter Hong Leong Cheah86% (7)
- QA Cations Lab-14Dokumen10 halamanQA Cations Lab-14Asim HandyBelum ada peringkat
- Reactions of Protein-01!11!2018Dokumen26 halamanReactions of Protein-01!11!2018Rdh MnbBelum ada peringkat
- Glucose Assay by Dinitrosalicylic Colorimetric MethodDokumen7 halamanGlucose Assay by Dinitrosalicylic Colorimetric MethodAbdullah Noordin50% (2)
- S Determination of Caffeine in BeveragesDokumen5 halamanS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- Potentiometric Titration Ex17Dokumen10 halamanPotentiometric Titration Ex17Tien HaminhBelum ada peringkat
- Module Anachem Acid-Base 1 PDFDokumen9 halamanModule Anachem Acid-Base 1 PDFarejay castro0% (1)
- Gel FiltrationDokumen5 halamanGel FiltrationRüveyda AkçinBelum ada peringkat
- Thin Layer ChromatographyDokumen2 halamanThin Layer ChromatographyOdessa Vidallon100% (3)
- Hazards of PolymersDokumen9 halamanHazards of PolymersTalha Shaukat67% (3)
- Enzyme KineticsDokumen8 halamanEnzyme KineticsyaivaziBelum ada peringkat
- Class Room Problems: Stoichiometry - Ii Page # 16Dokumen25 halamanClass Room Problems: Stoichiometry - Ii Page # 16Mary Grace Narvaez GarciaBelum ada peringkat
- EXP 2 RancidityDokumen6 halamanEXP 2 RancidityNur SyahirahBelum ada peringkat
- Enzyme LabDokumen11 halamanEnzyme Labjenh19167% (3)
- TLC Separation of Amino AcidsDokumen5 halamanTLC Separation of Amino Acidshmtlion0% (5)
- SDS-PAGE Electrophoresis of Unknown ProteinDokumen3 halamanSDS-PAGE Electrophoresis of Unknown Proteinjmario6660% (1)
- Determination of PH Exp No: 3 Date AimDokumen2 halamanDetermination of PH Exp No: 3 Date AimkuthappadyBelum ada peringkat
- Spinach Chromatography Lab 1Dokumen7 halamanSpinach Chromatography Lab 1api-392376456Belum ada peringkat
- Analysis of Aspirin LabDokumen2 halamanAnalysis of Aspirin LabBen GoodmanBelum ada peringkat
- MIC481 Lab ReportDokumen6 halamanMIC481 Lab ReportAbg Khairul Hannan Bin Abg AbdillahBelum ada peringkat
- Chem 132.2 - Biochemistry (Laboratory) Laboratory ReportDokumen4 halamanChem 132.2 - Biochemistry (Laboratory) Laboratory ReportCaryl Anne Dumdum CagaraBelum ada peringkat
- Experiment 5 Carbohydrate CharacterizationDokumen4 halamanExperiment 5 Carbohydrate CharacterizationPrince Robert Chua100% (1)
- Isolation of Genomic DNADokumen16 halamanIsolation of Genomic DNASamra KousarBelum ada peringkat
- Spectrophotometry Basic ConceptsDokumen7 halamanSpectrophotometry Basic ConceptsVon AustriaBelum ada peringkat
- CH102 Lab 5 Aldehydes and Ketones PDFDokumen10 halamanCH102 Lab 5 Aldehydes and Ketones PDFAnonymous caERsABelum ada peringkat
- Determination Acetic AcidDokumen21 halamanDetermination Acetic Acidameyakem100% (1)
- Formal Titration of Amino AcidDokumen5 halamanFormal Titration of Amino AcidMeyan Pratiwi100% (2)
- Exploring Chemical Analysis Self-TestDokumen30 halamanExploring Chemical Analysis Self-TestDavid Downing100% (1)
- Buffer TAE SpecificationsDokumen2 halamanBuffer TAE SpecificationsMuhammad Pawpaw FauziBelum ada peringkat
- Redox TitrationDokumen70 halamanRedox TitrationIlham Krisdarmawan Putra100% (1)
- Biochem 10A Lab QuestionsDokumen6 halamanBiochem 10A Lab QuestionsPaul A IBattledaily Scavella100% (1)
- (BIOCHEM LAB) CarbohydratesDokumen9 halaman(BIOCHEM LAB) Carbohydratesprettyfriends 05Belum ada peringkat
- BI309 Practical 6Dokumen8 halamanBI309 Practical 6SanahKumar100% (1)
- UNIT: SpectrophotometryDokumen15 halamanUNIT: SpectrophotometrybiddyusmcBelum ada peringkat
- Lab 6 Paper ChromatographyDokumen8 halamanLab 6 Paper Chromatographyapi-384770852Belum ada peringkat
- Onion Dna IsolationDokumen4 halamanOnion Dna IsolationSmriti SmreetiBelum ada peringkat
- University of ZimbabweDokumen7 halamanUniversity of ZimbabweronaldBelum ada peringkat
- 5 ChromatographyDokumen7 halaman5 ChromatographyAntonio CharismaBelum ada peringkat
- Chromatography of Amino Acids Lab ReportDokumen2 halamanChromatography of Amino Acids Lab ReportAjagwu EustaceBelum ada peringkat
- Chm421-Experiment 9 - Separation of Amino Acid Mixture by Paper ChromatographyDokumen8 halamanChm421-Experiment 9 - Separation of Amino Acid Mixture by Paper Chromatographynipale hiBelum ada peringkat
- ChromatographyDokumen11 halamanChromatographyAmrit KoiralaBelum ada peringkat
- Paper ChromatographyDokumen5 halamanPaper ChromatographyKarishmaBelum ada peringkat
- AOAC 2014.009 Pesticides (2022)Dokumen6 halamanAOAC 2014.009 Pesticides (2022)Miguel VBelum ada peringkat
- Kaufmann1996 Analysis of Carotenoids and CarotenolDokumen12 halamanKaufmann1996 Analysis of Carotenoids and CarotenolKirianova GodoyBelum ada peringkat
- 2.4.1 Toxi-A and Toxi-B Gc-Ms Rev 5Dokumen5 halaman2.4.1 Toxi-A and Toxi-B Gc-Ms Rev 5Mark ReinhardtBelum ada peringkat
- Astm E1840-1996 (2007)Dokumen3 halamanAstm E1840-1996 (2007)Ali MohsinBelum ada peringkat
- N - and C-Terminal Protein Sequencing by MALDI ISD Feb 2009Dokumen5 halamanN - and C-Terminal Protein Sequencing by MALDI ISD Feb 2009andrewmunk100% (2)
- Single Quadrupole Compact Mass Spectrometer For Liquid SampleDokumen6 halamanSingle Quadrupole Compact Mass Spectrometer For Liquid SampleJingwei LuoBelum ada peringkat
- Practical 1 Absorption SpectrosDokumen11 halamanPractical 1 Absorption SpectrosKjell CornelisBelum ada peringkat
- 13.14 C NMR SpectrosDokumen17 halaman13.14 C NMR SpectrosnanaBelum ada peringkat
- Flame Ionization Detector - Wikipedia, The Free EncyclopediaDokumen3 halamanFlame Ionization Detector - Wikipedia, The Free Encyclopediaandrew jachson labitoBelum ada peringkat
- Monograph - Diazepam SWGDRUGDokumen10 halamanMonograph - Diazepam SWGDRUGjavier chavezBelum ada peringkat
- Deslandres Table N2DataDokumen5 halamanDeslandres Table N2DataPiyali GhoshBelum ada peringkat
- Ima 22-23Dokumen4 halamanIma 22-23Archak SinghBelum ada peringkat
- Classification of SpectrosDokumen2 halamanClassification of SpectrosMD. DELWAR HOSSAINBelum ada peringkat
- 5977B Data Sheet1785Dokumen2 halaman5977B Data Sheet1785Guillermo HuertaBelum ada peringkat
- Dokumen - Tips X Ray Fluorescence Analysis Xrfnewucjinrruimgsectionsfileproject X RayDokumen4 halamanDokumen - Tips X Ray Fluorescence Analysis Xrfnewucjinrruimgsectionsfileproject X RayPrimawati RahmaniyahBelum ada peringkat
- Proceedings of Spie: Rapid, Automated, Quality Control of Diffraction Grating EfficiencyDokumen6 halamanProceedings of Spie: Rapid, Automated, Quality Control of Diffraction Grating EfficiencyironbatjediBelum ada peringkat
- Agilent Series 1100 LC-MSDDokumen12 halamanAgilent Series 1100 LC-MSDRosalio Velarde BarrazaBelum ada peringkat
- Instrumental Analysis IIDokumen3 halamanInstrumental Analysis IIashenafi100% (2)
- BBT - AssignmentDokumen3 halamanBBT - AssignmentalishbajohnBelum ada peringkat
- AAS Lab ManualDokumen3 halamanAAS Lab ManualJC100% (1)
- Problems Related To SpectrosDokumen4 halamanProblems Related To SpectrosAkshay KaushikBelum ada peringkat
- M. Douay Et Al - New Observations of The A 1-Pi-u-X 1-Sigma-G + Transition (Phillips System) of C2Dokumen11 halamanM. Douay Et Al - New Observations of The A 1-Pi-u-X 1-Sigma-G + Transition (Phillips System) of C2Damxz5Belum ada peringkat
- Chemy 310 Experiment 4Dokumen8 halamanChemy 310 Experiment 4Faisal MumtazBelum ada peringkat
- Spectroscopic Techniques and Applications: Dr. Abhilasha MishraDokumen10 halamanSpectroscopic Techniques and Applications: Dr. Abhilasha MishraABHILASHA MISHRABelum ada peringkat