MSE 230 HW1 (Due 01/14, 01/15) Spring 2010
Diunggah oleh
pumjlffoDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
MSE 230 HW1 (Due 01/14, 01/15) Spring 2010
Diunggah oleh
pumjlffoHak Cipta:
Format Tersedia
MSE 230
HW1 (due 01/14, 01/15)
Spring 2010
1. Soft drinks are marketed in containers made of aluminum, glass, and polyester. (a) Name the main classification of each of these materials. (b) For each of these materials, chose one property from the list below and explain briefly how it contributes to the performance of the material in this application. Properties: density, stiffness or elastic modulus, fracture strength, thermal conductivity, heat capacity, optical transparency, corrosion resistance.
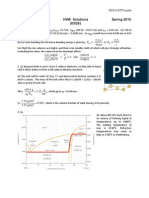
2. The table lists the worldwide annual production of selected materials on a mass basis (metric tons). (a) Complete the table by adding columns for the annual production on a volumetric basis and the values of the densities you use for the conversions. Cite your source of the density data and show one example calculation of the conversion. (b) Make a bar chart comparing the relative production on the two bases and comment on the results.
Material Steel Al-alloys Ti-alloys Polyethylene Polypropylene Wood Concrete Glass Brick
Annual Production (tonnes/yr) 9 x 108 2 x 107 9 x 104 6 x 107 4 x 107 2 x 109 2 x 1010 7 x 107 5 x 107
Density (kg/m3)
Annual Production (m3/year)
3. (a) Reproduce the table and add Elastic Linear Thermal columns for the melting temperature Metal Modulus Expansion Coefficient, (C) and crystal structure (fcc, bcc, (GPa) (m/mC) x 10-6 etc.); cite your source for these data. Al 70 23.1 (b) Make two plots of these data with Cu 100 16.5 clearly labeled axes: i) elastic Fe 210 11.8 modulus versus melting temperature Pb 14 28.9 (C) and ii) thermal expansion Mg 44 24.8 coefficient versus melting temperature Mo 330 4.8 (C). Label each data point with the Co 205 14.4 chemical symbol for the metal and its W 350 4.5 crystal structure. (c) Briefly explain the trends in terms of the bonding energy versus interatomic distance curve. 4. Based on x-ray diffraction measurements, we know that Ti has an hcp crystal structure with lattice parameters a = 0.29512 nm and c = 0.46845 nm. (a) Using this data calculate the density of perfect Ti crystal. (b) Compare this value with that reported for commercially pure Ti in the Appendix B in Callister and explain the slight difference.
Anda mungkin juga menyukai
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- 230 S10 HW11Dokumen1 halaman230 S10 HW11pumjlffoBelum ada peringkat
- MSE 230 HW12 (Due 04/22, 04/23) Spring 2010Dokumen1 halamanMSE 230 HW12 (Due 04/22, 04/23) Spring 2010pumjlffoBelum ada peringkat
- 230 S10 HW11 SolnsDokumen2 halaman230 S10 HW11 SolnspumjlffoBelum ada peringkat
- 230 S10 HW12 SolnsDokumen2 halaman230 S10 HW12 SolnspumjlffoBelum ada peringkat
- 230 S10 HW10 SolnsDokumen5 halaman230 S10 HW10 SolnspumjlffoBelum ada peringkat
- 230 S10 HW8Dokumen1 halaman230 S10 HW8pumjlffoBelum ada peringkat
- 230 S10 HW9Dokumen1 halaman230 S10 HW9pumjlffoBelum ada peringkat
- 230 S10 HW9 SolnsDokumen2 halaman230 S10 HW9 SolnspumjlffoBelum ada peringkat
- 230 S10 HW8 Solns0Dokumen3 halaman230 S10 HW8 Solns0pumjlffoBelum ada peringkat
- 230 S10 HW10Dokumen2 halaman230 S10 HW10pumjlffoBelum ada peringkat
- 230 S10 HW7 SolnsDokumen3 halaman230 S10 HW7 SolnspumjlffoBelum ada peringkat
- 230 S10 HW7Dokumen2 halaman230 S10 HW7pumjlffoBelum ada peringkat
- 230 S10 HW6Dokumen1 halaman230 S10 HW6pumjlffoBelum ada peringkat
- 230 S10 HW5Dokumen1 halaman230 S10 HW5pumjlffoBelum ada peringkat
- 230 S10 HW5 SolutionsDokumen2 halaman230 S10 HW5 SolutionspumjlffoBelum ada peringkat
- 230 S10 HW6 Solns0Dokumen3 halaman230 S10 HW6 Solns0pumjlffoBelum ada peringkat
- 230 S10 HW1 Solns0Dokumen3 halaman230 S10 HW1 Solns0pumjlffoBelum ada peringkat
- MSE 230 HW4 (Due 02/4, 02/5) Spring 2010Dokumen1 halamanMSE 230 HW4 (Due 02/4, 02/5) Spring 2010pumjlffoBelum ada peringkat
- 230 S10 HW3 Solutions0Dokumen5 halaman230 S10 HW3 Solutions0pumjlffoBelum ada peringkat
- 230 S10 HW3Dokumen1 halaman230 S10 HW3pumjlffoBelum ada peringkat
- 230 S10 HW4 SolutionsDokumen2 halaman230 S10 HW4 SolutionspumjlffoBelum ada peringkat
- 230 S10 HW2 SolnsDokumen4 halaman230 S10 HW2 SolnspumjlffoBelum ada peringkat
- 230 S10 HW2Dokumen1 halaman230 S10 HW2pumjlffoBelum ada peringkat