Pelvic Inflammatory Disease (PID): Causes, Symptoms and Treatment
Diunggah oleh
Alfieyanto SyaripuddinDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Pelvic Inflammatory Disease (PID): Causes, Symptoms and Treatment
Diunggah oleh
Alfieyanto SyaripuddinHak Cipta:
Format Tersedia
Pelvic Inflammatory Disease.
(PID)
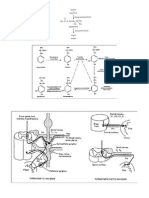
PID : an infectious and inflammatory disorder of the upper female reproductive tract, including the uterus, fallopian tubes, and adjacent pelvic structures. PID is initiated by infection that ascends from the vagina and cervix. Chlamydia trachomatis is the predominant sexually transmitted organism causing PID At presentation, women with PID may range from asymptomatic to seriously ill. The most common presenting complaint is lower abdominal pain. Many women also exhibit an abnormal vaginal discharge. The diagnosis of acute PID is primarily based on historical and clinical findings, but many patients may exhibit only a few or no symptoms.
Laparoscopy is the current criterion standard for the diagnosis of PID. No single test is highly specific or sensitive for the disease. Empirical treatment is suggested by the Centers for Disease Control and Prevention (CDC) Sexually Transmitted Disease Management Guidelines in patients with uterine or adnexal tenderness and cervical motion tenderness, if no other etiology explains the findings. All antibiotic regimens must be effective against C trachomatis and N gonorrhoeae, as well as against gram-negative facultative organisms, anaerobes, and streptococci . Most patients are now treated in an outpatient setting, but physicians should consider hospitalization in selected cases
"Violin-string" adhesions of chronic Fitz-Hugh-Curtis syndrome.
Alfie.S
Anatomy. PID may extend from infection of the lower female reproductive tract, including the vagina and cervix. PID is an infectious and inflammatory disorder of the upper female reproductive tract, including the uterus and fallopian tubes. Infection and inflammation may spread to adjacent pelvic structures in the pelvis and abdomen, including perihepatic structures (Fitz-Hugh Curtis syndrome).
Pathophysiology. Most cases of PID are presumed to occur in 2 stages. 1. The first stage is acquisition of a vaginal or cervical infection; this infection is often sexually transmitted and may be asymptomatic. 2. The second stage is direct ascent of microorganisms from the vagina or cervix to the upper genital tract, with infection and inflammation of these structures. The exact mechanism of ascent of microorganisms from the vagina and cervix is unknown.
1. Although cervical mucus provides a functional barrier against upward spread, the efficacy of this mechanism may be decreased by hormonal changes that occur during ovulation and menstruation. 2. Alterations in the cervicovaginal microenvironment may also result from antibiotic treatment and sexually transmitted infections that can disrupt the balance of endogenous flora, causing normally nonpathogenic organisms to overgrow and ascend. 3. Opening of the cervix during menstruation with retrograde menstrual flow may also facilitate ascent of microorganisms. 4. Intercourse may contribute to the ascent of infection due to rhythmic mechanical uterine contractions. Bacteria may be carried along with sperm into the uterus and tubes 5. Genetic polymorphisms of PID pathogens affect the likelihood that a lower tract infection will progress to frank PID. Chlamydial heat shock protein 60 (CHSP60) antigen expression in C trachomatis and P9Opa(b) protein expression in N gonorrhoeae are examples of specific bacterial genes implicated in the pathology of PID. In the upper tract, a number of microbial and host factors appear to play a role in the degree of host inflammation and resultant scarring.
Tubal infection initially affects the mucosa, but acute, complement-mediated transmural inflammation may develop rapidly and increase in intensity with subsequent infections. Inflammation may extend to uninfected parametrial structures, including the bowel. Infection may extend by 1. Spillage of purulent materials from the fallopian tubes 2. Lymphatic spread beyond the pelvis to produce acute peritonitis and acute perihepatitis (Fitz-Hugh Curtis syndrome). Infecting organisms The organisms most commonly isolated in many, if not most, cases of acute PID are 1. Neisseria gonorrhoeae 2. Chlamydia trachomatis C trachomatis, an intracellular bacterial pathogen, is the predominant sexually transmitted organism causing PID However, newer studies using more sensitive and specific laparoscopic cultures have found acute PID to be polymicrobial in up to 30-40% of cases. N gonorrhoeae and C trachomatis may be instrumental in the initial infection of the upper tract, with anaerobes, facultative anaerobes, and
other bacteria increasingly isolated as inflammation increases and abscesses form. Organisms involved include the following:
Gardnerella vaginalis Mycoplasma hominis Mycoplasma genitalium[ Ureaplasma urealyticum Herpes simplex virus2 (HSV-2) Trichomonas vaginalis Cytomegalovirus Haemophilus influenza Streptococcus agalactiae Enteric gram-negative rods (Escherichia coli) Peptococcus species Anaerobes
In addition cytomegalovirus (CMV) has been found in the upper genital tracts of women with PID, suggesting a potential role of CMV in PID. In iatrogenically induced infections, the endogenous microflora of the vagina predominate. Bacteroides fragilis can cause tubal and epithelial destruction. The microbiology of PID has also been found to reflect the predominant sexually transmitted infections (STIs) prevalent within a specific population and also less-common organisms seen in that population.
Bacterial vaginosis (BV) is suggested to play a role in the initiation of ascending infection in a subset of women with heavy growth of BV-associated organisms, such as G vaginalis, more than 2 recent sexual partners, and especially after recent abortion or gynecologic surgery. In less-developed countries, PID may be due to a granulomatous salpingitis caused by Mycobacterium tuberculosis or Schistosoma species Co-infection of HSV-2 with N gonorrhoeae, C trachomatis, and BV was also associated with histologic evidence of acute endometritis. HSV-2 was demonstrated to be associated with fallopian tube inflammation and lower tract ulcerations that may contribute to disruption of the endocervical canal mucus barrier HIV infection has been found to be associated with an increased incidence of C trachomatis infection, Candida, and human papillomavirus. Women with HIV infection also have an increased risk of progression to PID and tubo-ovarian abscess. Microbial virulence appears to play a significant role in PID.
Risk factors
Risk factors for PID include 1. multiple sexual partners, 2. a history of prior STIs 3. a history of sexual abuse. Frequent vaginal douching has also been implicated. 4. Younger age has been found to be associated with increased risk, suggested to be due to some combination of increased cervical mucosal permeability, a larger zone of cervical ectopy, a lower prevalence of protective chlamydial antibodies, and increased risk-taking behaviors. 5. Surgical procedures, such as endometrial biopsy, curettage, and hysteroscopies break the cervical barrier, predisposing women to ascending infections.
Alfie.S
Anda mungkin juga menyukai
- Contagious Diseases: The Science, History, and Future of Epidemics. From Ancient Plagues to Modern Pandemics, How to Stay Ahead of a Global Health CrisisDari EverandContagious Diseases: The Science, History, and Future of Epidemics. From Ancient Plagues to Modern Pandemics, How to Stay Ahead of a Global Health CrisisBelum ada peringkat
- Pelvic Inflammatory Disease - 1Dokumen8 halamanPelvic Inflammatory Disease - 1fatqur28Belum ada peringkat
- Viral Vistas: Insights into Infectious Diseases: The Invisible War: Decoding the Game of Hide and Seek with PathogensDari EverandViral Vistas: Insights into Infectious Diseases: The Invisible War: Decoding the Game of Hide and Seek with PathogensBelum ada peringkat
- Monografia de PelvicDokumen12 halamanMonografia de PelvicTracyVillegasBelum ada peringkat
- Pelvic Inflammatory DiseaseDokumen8 halamanPelvic Inflammatory DiseaseAndyan Adlu Prasetyaji0% (1)
- Lectie PIDDokumen59 halamanLectie PIDNatalie BondariBelum ada peringkat
- PathophysiologyDokumen5 halamanPathophysiologyJessyl GirayBelum ada peringkat
- Pelvic Inflammatory DiseaseDokumen7 halamanPelvic Inflammatory DiseaseMephisto D. LeffertlarkBelum ada peringkat
- Pelvic Inflammatory Disease Cause Infertility: A Condition Requiring Closer AttentionDokumen60 halamanPelvic Inflammatory Disease Cause Infertility: A Condition Requiring Closer AttentionVergina ClaudiaBelum ada peringkat
- Pelvic Inflammatory DiseaseDokumen18 halamanPelvic Inflammatory DiseaseVictorBelum ada peringkat
- Tratamiento EpiDokumen13 halamanTratamiento EpiFrankRodríguezLuisBelum ada peringkat
- (000043) PDFDokumen30 halaman(000043) PDFedi_wsBelum ada peringkat
- Pelvic Inflamatory DiseaseDokumen8 halamanPelvic Inflamatory Diseasetam mei100% (1)
- Sexually Transmitted DiseasesDokumen71 halamanSexually Transmitted DiseasesAkwu AkwuBelum ada peringkat
- Pelvic Inflammatory Disease and Tubo-ovarian AbscessDokumen16 halamanPelvic Inflammatory Disease and Tubo-ovarian AbscessKaye VictorianoBelum ada peringkat
- TORCH Infection GuideDokumen35 halamanTORCH Infection GuideGerarld Immanuel KairupanBelum ada peringkat
- Clinical Manifestations and Diagnosis of Chlamydia Trachomatis InfectionsDokumen28 halamanClinical Manifestations and Diagnosis of Chlamydia Trachomatis InfectionsAmbarBelum ada peringkat
- BACTERIAL INFECTIONS: CAUSES AND SYMPTOMS OF PELVIC INFLAMMATORY DISEASEDokumen5 halamanBACTERIAL INFECTIONS: CAUSES AND SYMPTOMS OF PELVIC INFLAMMATORY DISEASEratuauliarsr xBelum ada peringkat
- Cervicitis Causes, Symptoms, Diagnosis and TreatmentDokumen7 halamanCervicitis Causes, Symptoms, Diagnosis and TreatmentElaisa Mae Delos SantosBelum ada peringkat
- Pid PDFDokumen4 halamanPid PDFNilakshi Barik MandalBelum ada peringkat
- Reproductive Tract Infections: An Introductory Overview: Types of InfectionDokumen30 halamanReproductive Tract Infections: An Introductory Overview: Types of InfectionSherlocknovBelum ada peringkat
- Female Genital Inflammatory DiseasesDokumen39 halamanFemale Genital Inflammatory DiseaseseveeBelum ada peringkat
- Infections of The Digestive GlandsDokumen17 halamanInfections of The Digestive GlandsCrystal Lynn Keener SciariniBelum ada peringkat
- PID: Understanding Pelvic Inflammatory DiseaseDokumen34 halamanPID: Understanding Pelvic Inflammatory Diseaseraed faisalBelum ada peringkat
- Neisseria Gonorrhoeae, The Gonococcus, Is A Gram-Negative Diplococcus Which Causes TheDokumen2 halamanNeisseria Gonorrhoeae, The Gonococcus, Is A Gram-Negative Diplococcus Which Causes TheSunny OZBelum ada peringkat
- Pelvic Inflammatory Disease: Causes, Symptoms, Diagnosis and TreatmentDokumen5 halamanPelvic Inflammatory Disease: Causes, Symptoms, Diagnosis and TreatmentGina Eliana Custodio GonzalesBelum ada peringkat
- First Page PDFDokumen1 halamanFirst Page PDFNur intan cahyaniBelum ada peringkat
- Pelvic Inflammatory Disease (Pid)Dokumen42 halamanPelvic Inflammatory Disease (Pid)Aruna PoovalingamBelum ada peringkat
- Pelvic Inflammatory Disease: Tori Hudson, NDDokumen8 halamanPelvic Inflammatory Disease: Tori Hudson, NDFranciscus BuwanaBelum ada peringkat
- Sexually Transmitted Causes of Urethritis, Proctitis, Pharyngitis and CervicitisDokumen7 halamanSexually Transmitted Causes of Urethritis, Proctitis, Pharyngitis and CervicitismingBelum ada peringkat
- Bacteسيبليسليسl genital tract infectionsDokumen60 halamanBacteسيبليسليسl genital tract infectionsDr.Issam JumaaBelum ada peringkat
- Sexually Transmitted DiseasesDokumen132 halamanSexually Transmitted DiseasesanitacharisBelum ada peringkat
- Dr. Eka's Guide to Sexually Transmitted InfectionsDokumen67 halamanDr. Eka's Guide to Sexually Transmitted Infectionsuut14Belum ada peringkat
- Pelvic Inflammatory Disease GuideDokumen5 halamanPelvic Inflammatory Disease GuideRahma Rafina100% (2)
- Vaginitis EmedicineDokumen17 halamanVaginitis EmedicineIndah HaneBelum ada peringkat
- CURS ENGLEZA Boli InfectioaseDokumen246 halamanCURS ENGLEZA Boli InfectioaseKiran LetrangeBelum ada peringkat
- Study Notes For Sexually Transmitted DiseasesDokumen4 halamanStudy Notes For Sexually Transmitted DiseasesPrince K. TaileyBelum ada peringkat
- Acute CervicitisDokumen9 halamanAcute CervicitisVicobeingoBelum ada peringkat
- Sti RtiDokumen88 halamanSti RtiDr.L.R.Ahirwar100% (1)
- Boushra Rahman Postpartum InfectionDokumen1 halamanBoushra Rahman Postpartum InfectionClinica GinecologicaBelum ada peringkat
- Pid BSTDokumen17 halamanPid BSTPutri Senna RahayuBelum ada peringkat
- Chorioamnionitis: From Pathogenesis To Treatment: ReviewDokumen8 halamanChorioamnionitis: From Pathogenesis To Treatment: ReviewSri MardLaniahBelum ada peringkat
- Neisseria gonorrhoeae (GonococcusDokumen28 halamanNeisseria gonorrhoeae (GonococcusRoni Ananda Perwira HarahapBelum ada peringkat
- Infeksi Genitalia Interna & ExternaDokumen63 halamanInfeksi Genitalia Interna & ExternaPoetri IermayaniBelum ada peringkat
- Chorio TXDokumen8 halamanChorio TXnurul azareeBelum ada peringkat
- Case StudyDokumen5 halamanCase StudyDeo Micah GoBelum ada peringkat
- Acute Cervicitis - UpToDateDokumen28 halamanAcute Cervicitis - UpToDatePatriciaBelum ada peringkat
- SOCHINF (2009) Diagnostico ITS No Virales - Parte 1Dokumen11 halamanSOCHINF (2009) Diagnostico ITS No Virales - Parte 1Esteban OrellanaBelum ada peringkat
- Dr. Omnictin's guide to infectious diseases in pregnancyDokumen15 halamanDr. Omnictin's guide to infectious diseases in pregnancyroselo alagaseBelum ada peringkat
- Abstract: Gonorrhea Is A Set of Clinical Conditions Resulting From Infection WithDokumen5 halamanAbstract: Gonorrhea Is A Set of Clinical Conditions Resulting From Infection WithLaili Firda RusdianaBelum ada peringkat
- Gyne - (Section B) PID-STI-1Dokumen45 halamanGyne - (Section B) PID-STI-1Kailash KhatriBelum ada peringkat
- Herpes JurnalDokumen6 halamanHerpes Jurnalags.suryono102Belum ada peringkat
- Differential Diagnosis of Painful Genital LesionsDokumen7 halamanDifferential Diagnosis of Painful Genital LesionsKyra KhalidBelum ada peringkat
- Ets SexualDokumen18 halamanEts SexualwilliamBelum ada peringkat
- Female Reproductive System Disorders and InfectionsDokumen14 halamanFemale Reproductive System Disorders and InfectionsazwararifkiBelum ada peringkat
- Diphtheria: About The DiseaseDokumen18 halamanDiphtheria: About The DiseaseJesse EstradaBelum ada peringkat
- Sexually Transmitted Infections (Sti) Ii: Return To SyllabusDokumen15 halamanSexually Transmitted Infections (Sti) Ii: Return To SyllabusANGGITA LARASATI PURBANINGRUMBelum ada peringkat
- The Infectious Disease Process-2Dokumen6 halamanThe Infectious Disease Process-2Ruchi SharmaBelum ada peringkat
- A Review On The Diagnosis and Treatment of SyphiliDokumen8 halamanA Review On The Diagnosis and Treatment of SyphiliRaffaharianggaraBelum ada peringkat
- Pelvic Inflammatory Disease - Clinical Manifestations and DiagnosisDokumen13 halamanPelvic Inflammatory Disease - Clinical Manifestations and DiagnosisJavier Manuel Escobedo CalderónBelum ada peringkat
- New Schedule For Surgery PostingDokumen1 halamanNew Schedule For Surgery PostingAlfieyanto SyaripuddinBelum ada peringkat
- PBL Week4 PDFDokumen1 halamanPBL Week4 PDFAlfieyanto SyaripuddinBelum ada peringkat
- PBLDokumen3 halamanPBLAlfieyanto SyaripuddinBelum ada peringkat
- MC TextDokumen2 halamanMC TextAlfieyanto Syaripuddin100% (3)
- String length recommendations and brace height advice for Uukha bowsDokumen1 halamanString length recommendations and brace height advice for Uukha bowsPak Cik FauzyBelum ada peringkat
- Hidaat Alem The Medical Rights and Reform Act of 2009 University of Maryland University CollegeDokumen12 halamanHidaat Alem The Medical Rights and Reform Act of 2009 University of Maryland University Collegepy007Belum ada peringkat
- Future42 1675898461Dokumen48 halamanFuture42 1675898461Rodrigo Garcia G.Belum ada peringkat
- Spelling Errors Worksheet 4 - EditableDokumen2 halamanSpelling Errors Worksheet 4 - EditableSGillespieBelum ada peringkat
- Gambaran Kebersihan Mulut Dan Karies Gigi Pada Vegetarian Lacto-Ovo Di Jurusan Keperawatan Universitas Klabat AirmadidiDokumen6 halamanGambaran Kebersihan Mulut Dan Karies Gigi Pada Vegetarian Lacto-Ovo Di Jurusan Keperawatan Universitas Klabat AirmadidiPRADNJA SURYA PARAMITHABelum ada peringkat
- Volvo FM/FH with Volvo Compact Retarder VR 3250 Technical DataDokumen2 halamanVolvo FM/FH with Volvo Compact Retarder VR 3250 Technical Dataaquilescachoyo50% (2)
- MCI FMGE Previous Year Solved Question Paper 2003Dokumen0 halamanMCI FMGE Previous Year Solved Question Paper 2003Sharat Chandra100% (1)
- Centre's Letter To States On DigiLockerDokumen21 halamanCentre's Letter To States On DigiLockerNDTVBelum ada peringkat
- Shahin CVDokumen2 halamanShahin CVLubainur RahmanBelum ada peringkat
- Dyson Case StudyDokumen4 halamanDyson Case Studyolga100% (3)
- Sample PPP Lesson PlanDokumen5 halamanSample PPP Lesson Planapi-550555211Belum ada peringkat
- 100 Bedded Hospital at Jadcherla: Load CalculationsDokumen3 halaman100 Bedded Hospital at Jadcherla: Load Calculationskiran raghukiranBelum ada peringkat
- What Is Innovation and Its Characteristics of InnovationDokumen4 halamanWhat Is Innovation and Its Characteristics of InnovationMohd TauqeerBelum ada peringkat
- FraudDokumen77 halamanFraudTan Siew Li100% (2)
- NetZoom Pro v15 Install GuideDokumen81 halamanNetZoom Pro v15 Install Guidescribd!!Belum ada peringkat
- Inver Powderpaint SpecirficationsDokumen2 halamanInver Powderpaint SpecirficationsArun PadmanabhanBelum ada peringkat
- SyllabusDokumen8 halamanSyllabusrickyangnwBelum ada peringkat
- Tomato & Tomato Products ManufacturingDokumen49 halamanTomato & Tomato Products ManufacturingAjjay Kumar Gupta100% (1)
- Network Profiling Using FlowDokumen75 halamanNetwork Profiling Using FlowSoftware Engineering Institute PublicationsBelum ada peringkat
- Dreams FinallDokumen2 halamanDreams FinalldeeznutsBelum ada peringkat
- The Best Chess BooksDokumen3 halamanThe Best Chess BooksJames Warren100% (1)
- License Key Windows 8Dokumen7 halamanLicense Key Windows 8Juned FahriBelum ada peringkat
- Henry VII Student NotesDokumen26 halamanHenry VII Student Notesapi-286559228Belum ada peringkat
- Compund and Complex Sentences ExerciseDokumen3 halamanCompund and Complex Sentences ExerciseTimothyBelum ada peringkat
- Prep - VN: Where Did The Polo Family Come From?Dokumen1 halamanPrep - VN: Where Did The Polo Family Come From?Phương LanBelum ada peringkat
- Factors Affecting Job Satisfaction of EngineersDokumen35 halamanFactors Affecting Job Satisfaction of Engineerslingg8850% (2)
- Jillian's Student Exploration of TranslationsDokumen5 halamanJillian's Student Exploration of Translationsjmjm25% (4)
- Shakespeare's Sonnets Portray a Deeper Understanding of Authentic Love Compared to Marlowe's Idealistic PerspectiveDokumen3 halamanShakespeare's Sonnets Portray a Deeper Understanding of Authentic Love Compared to Marlowe's Idealistic Perspectivemaria blascosBelum ada peringkat
- Cookery-10 LAS-Q3 Week5Dokumen7 halamanCookery-10 LAS-Q3 Week5Angeline Cortez100% (1)
- Safety Moment Manual LiftingDokumen1 halamanSafety Moment Manual LiftingEvert W. VanderBerg100% (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDari EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionPenilaian: 4 dari 5 bintang4/5 (402)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDari EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityPenilaian: 4 dari 5 bintang4/5 (13)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDari EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsBelum ada peringkat
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDari EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeBelum ada peringkat
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDari EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsPenilaian: 3.5 dari 5 bintang3.5/5 (3)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDari EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedPenilaian: 5 dari 5 bintang5/5 (78)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDari EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- Breaking the Habit of Being YourselfDari EverandBreaking the Habit of Being YourselfPenilaian: 4.5 dari 5 bintang4.5/5 (1454)
- How to Talk to Anyone: Learn the Secrets of Good Communication and the Little Tricks for Big Success in RelationshipDari EverandHow to Talk to Anyone: Learn the Secrets of Good Communication and the Little Tricks for Big Success in RelationshipPenilaian: 4.5 dari 5 bintang4.5/5 (1135)
- The Obesity Code: Unlocking the Secrets of Weight LossDari EverandThe Obesity Code: Unlocking the Secrets of Weight LossPenilaian: 5 dari 5 bintang5/5 (3)
- Deep Sleep Hypnosis: Guided Meditation For Sleep & HealingDari EverandDeep Sleep Hypnosis: Guided Meditation For Sleep & HealingPenilaian: 4.5 dari 5 bintang4.5/5 (103)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisDari EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisPenilaian: 4 dari 5 bintang4/5 (1)
- The Happiness Trap: How to Stop Struggling and Start LivingDari EverandThe Happiness Trap: How to Stop Struggling and Start LivingPenilaian: 4 dari 5 bintang4/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsDari EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsPenilaian: 4.5 dari 5 bintang4.5/5 (169)
- The Comfort of Crows: A Backyard YearDari EverandThe Comfort of Crows: A Backyard YearPenilaian: 4.5 dari 5 bintang4.5/5 (23)
- Techniques Exercises And Tricks For Memory ImprovementDari EverandTechniques Exercises And Tricks For Memory ImprovementPenilaian: 4.5 dari 5 bintang4.5/5 (40)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDari EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingPenilaian: 5 dari 5 bintang5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesDari EverandThe Ultimate Guide To Memory Improvement TechniquesPenilaian: 5 dari 5 bintang5/5 (34)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsDari EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsBelum ada peringkat
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDari EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingPenilaian: 3.5 dari 5 bintang3.5/5 (31)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessDari EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessPenilaian: 4.5 dari 5 bintang4.5/5 (327)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisDari EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisPenilaian: 5 dari 5 bintang5/5 (3)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDari EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifePenilaian: 4.5 dari 5 bintang4.5/5 (253)