Go 3 Alcohol, Ether, Amines
Diunggah oleh
cikaifaDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Go 3 Alcohol, Ether, Amines
Diunggah oleh
cikaifaHak Cipta:
Format Tersedia
ALCOHOL, ETHER & AMINES
Definition of alcohol
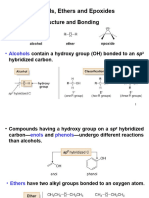
An alcohol is any organic compound in which a hydroxyl functional group (-OH) is bound to a carbon atom, usually connected to other carbon or hydrogen atoms
Definition of ether
Ethers ( /ir/) are a class of organic compounds that contain an ether group an oxygen atom connected to two alkyl or aryl groups of general formula ROR'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (CH3-CH2-O-CH2-CH3)
Definition of amines
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, where in one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group
Physical and Chemical Properties of Alcohol
Have an odor that often described as 'biting' and as 'hanging' in nasal passages. Protic solvents
The hydroxyl group makes the alcohol molecule polar. Those groups can form hydrogen bonds to one another and to other compounds (except in certain large molecules where the hydroxyl is protected by steric hindrance of adjacent groups.
Two opposing solubility trends in alcohols
The tendency of the polar OH to promote solubility in water The tendency of the carbon chain to resist it
All simple alcohols are miscible in organic solvents
Have higher boiling points than comparable gydrocarbon and ethers because of hydrogen bonding
The boiling point of the alcohol ethanol is 78.29 C, compared to 69 C for the hydrocarbon Hexane (a common constituent of gasoline), and 34.6 C for Diethyl ether
Can show either acidic or basic properties at the O-H group
Slightly weaker acids than water but still able to react with strong bases such as Sodium Hydride or reactive metals such as Sodium Salts that results are called alkoxides, with the general formula RO- M+
The oxygen atom has lone pairs of nonbonded electrons that render it weakly basic in the presence of strong acids such as sulfuric acids
Eg: Methanol
Preparation of Alcohol
Substitution
Primary alkyl halides react with aqueous NaOH or KOH mainly to primary alcohols in nucleophilic aliphatic substitution. (Secondary and especially tertiary alkyl halides will give the elimination (alkene) product instead). Grignard reagents react with carbonyl groups to secondary and tertiary alcohols. Related reactions are the Barbier reaction and the Nozaki-Hiyama reaction.
Reduction
Aldehydes or ketones are reduced with sodium borohydride or lithium aluminium hydride (after an acidic workup). Another reduction by aluminiumisopropylates is the Meerwein-Ponndorf-Verley reduction. Noyori asymmetric hydrogenation is the asymmetric reduction of -keto-esters.
Hydrolysis
Alkenes engage in an acid catalysed hydration reaction using concentrated sulfuric acid as a catalyst that gives usually secondary or tertiary alcohols. The hydroboration-oxidation and oxymercurationreduction of alkenes are more reliable in organic synthesis. Alkenes react with NBS and water in halohydrin formation reaction. Amines can be converted to diazonium salts, which are then hydrolyzed.
Application of alcohols
Ethylene glycol Alcohol fuel (mainly ethanol and methanol) Reagents or solvents
As an antifreeze
Because of low toxicity and ability to dissolve non-polar substances Ethanol is used as a solvent in medical drugs, perfumes, and vegetable essencess such as vanilla Organic synthesis - serve as versatile intermediates
Application of alcohols
Antiseptic
Disinfect the skin before injections are given, often along with iodine Not require drying due to volatility of the compound
Ethanol-based soaps Preservative for speciments
Alcohol gels
hand sanitizers
Physical properties of Ether
Ether molecules cannot form hydrogen bonds with each other
relatively low boiling points compared to the analogous alcohols
In the boiling points of the ethers and their isometric alcohols becomes lower as the carbon chains become longer
Because the van der Waals interactions of the extended carbon chain dominates over the presence of hydrogen bonding
Slightly polar
The C-O-C bond angle in the functional group is about 110, and the C-O dipoles do not cancel out
Ethers are more polar than alkenes but not as polar as alcohols, esters, or amides of comparable structure
However, the presence of two lone pairs of electrons on the oxygen atoms makes hydrogen bonding with water molecules possible
Cyclic ethers such as tetrahydrofuran and 1,4-dioxane are miscible in water
Because of the more exposed oxygen atom for hydrogen bonding as compared to aliphatic ethers
Reactions of Ether
Ether cleavage
Although ethers resist hydrolysis, they are cleaved by mineral acids such as hydrobromic acid and hydroiodic acid. Hydrogen chloride cleaves ethers only slowly Methyl ethers typically afford methyl halides: ROCH3 + HBr CH3Br + ROH These reactions proceed via onium intermediates, i.e. [RO(H)CH3]+Br Some ethers rapidly cleave with boron tribromide (even aluminium chloride is used in some cases) to give the alkyl bromide Depending on the substituents, some ethers can be cleaved with a variety of reagents, e.g. strong base.
Peroxide formation
When stored in the presence of air or oxygen, ethers tend to form explosive peroxides, such as diethyl ether peroxide The reaction is accelerated by light, metal catalysts, and aldehydes In addition to avoiding storage conditions likely to form peroxides, it is recommended, when an ether is used as a solvent, not to distill it to dryness, as any peroxides that may have formed, being less volatile than the original ether, will become concentrated in the last few drops of liquid.
Lewis bases
Ethers serve as Lewis bases and Bronsted bases Strong acids protonate the oxygen to give "onium ions." For instance, diethyl ether forms a complex with boron trifluoride, i.e. diethyl etherate (BF3.OEt2) Ethers also coordinate to Mg(II) center in Grignard reagents Polyethers, including many antibiotics, cryptands, and crown ethers, bind alkali metal cations strongly.
Alphahalogenation
This reactivity is akin to the tendency of ethers with alpha hydrogen atoms to form peroxides Chlorine gives alpha-chloroethers.
Preparation of Ether
Dehydration of alcohols
The Dehydration of alcohols affords ethers: 2 R-OH R-O-R + H2O at high temperature This direct reaction requires elevated temperatures (about 125 C) The reaction is catalyzed by acids, usually sulfuric acid The method is effective for generating symmetrical ethers, but not unsymmetrical ethers Diethyl ether is produced from ethanol by this method Cyclic ethers are readily generated by this approach. Such reactions must compete with dehydration of the alcohol: R-CH2-CH2(OH) R-CH=CH2 + H2O The dehydration route often requires conditions incompatible with delicate molecules Nucleophilic displacement of alkyl halides by alkoxides : R-ONa + R'-X R-O-R' + NaX This reaction is called the Williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alkoxide, followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R-X) Suitable leaving groups (X) include iodide, bromide, or sulfonates This method usually does not work well for aryl halides (e.g. bromobenzene (see Ullmann condensation below) Likewise, this method only gives the best yields for primary halides. Secondary and tertiary halides are prone to undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction due to steric hindrance from the large alkyl groups. In a related reaction, alkyl halides undergo nucleophilic displacement by phenoxides The R-X cannot be used to react with the alcohol However, phenols can be used to replace the alcohol, while maintaining the alkyl halide. Since phenols are acidic, they readily react with a strong base like sodium hydroxide to form phenoxide ions The phenoxide ion will then substitute the -X group in the alkyl halide, forming an ether with an aryl group attached to it in a reaction with an SN2 mechanism : C6H5OH + OH- C6H5-O- + H2O : C6H5-O- + R-X C6H5OR The Ullmann condensation is similar to the Williamson method except that the substrate is an aryl halide Such reactions generally require a catalyst, such as copper. Alcohols add to electrophilically activated alkenes : R2C=CR2 + R-OH R2CH-C(O-R)-R2 Acid catalysis is required for this reaction. Often, mercury trifluoroacetate (Hg(OCOCF3)2) is used as a catalyst for the reaction, geneating an ether with Markovnikov regiochemistry Using similar reactions, tetrahydropyranyl ethers are used as protective groups for alcohols.
Williamson ether synthesis
Ullmann condensation
Electrophilic addition of alcohols to alkenes
Preparation of epoxides
Epoxides are typically prepared by oxidation of alkenes The most important epoxide in terms of industrial scale is ethylene oxide, which is produced by oxidation of ethylene with oxygen Other epoxides are produced by one of two routes: By the oxidation of alkenes with a peroxyacid such as m-CPBA. By the base intramolecular nucleophilic substitution of a halohydrin.
Classes of amines
Aliphatic amines
Primary alkyl amines include methylamine, ethanolamine (2-aminoethanol), and the buffering agent tris
Aromatic amines
Aromatic amines have the nitrogen atom connected to an aromatic ring as in anilines
Secondary amines have two alkyl substituents bound to N together with one hydrogen. Important representatives include dimethylamine and methy lethanolamine
The aromatic ring decreases the alkalinity of the amine, depending on its substituents
In tertiary amines, all three hydrogen atoms are replaced by organic substituents
The presence of an amine group strongly increases the reactivity of the aromatic ring, due to an electron-donating effect.
Physical properties of Amines
The boiling point of amines is higher than those of the corresponding phosphines, but generally lower than those of the corresponding alcohols
Methylamine and ethylamine are gases under standard conditions
Methyl alcohol and ethyl alcohols are liquid in standard conditions
Physical properties of Amines
Most aliphatic amines display some solubility in water
Solubility decreases with the increase in the number of carbon atoms
Aliphatic amines display significant solubility in organic solvents, especially polar organic solvents
Primary amines react with Ketones such as acetones
Application of amines
Dyes
Application of amines
Drugs
Gas treatment
Dyes
Primary aromatic amines are used as a starting material for the manufacture of azo dyes. It reacts with nitric(III) acid to form diazonium salt, which can undergo coupling reaction to form azo compound. As azo-compounds are highly coloured, they are widely used in dyeing industries
Methyl orange
Direct Brown 138
Sunset Yellow
Ponceau
Drugs
Chlorpheniramine
An antihistamine that helps to relieve allergic disorders due to cold, hay fever, itchy skin, insect bites and stings.
Chlorpromazine
A tranquillizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder.
Ephedrine and Phenylephrine
, as amine hydrochlorides, are used as decongestants.
Amphetamine, Methamphetamine, and Methcathinone
are psychostimulant amines that are listed as controlled substances by the US DEA.
Amitriptyline, Imipramine, Lofepramine and Clomipramine
are tricyclic antidepressants and tertiary amines.
Nortriptyline, Desipramine, and Amoxapine
are tricyclic antidepressants and secondary amines. (The tricyclics are grouped by the nature of the final amine group on the side chain.)
Gas treatment
- Aqueous monoethanolamine (MEA) - diglycolamine (DGA) - diethanolamine (DEA) - diisopropanolamine (DIPA) - methyldiethanolamine (MDEA)
- removing carbon dioxide(CO2) and hydrogen sulfide (H2S) from natural gas - refinery process streams - remove CO2 from combustion gases / flue gases - may have potential for abatement ofgreenhouse gases
Anda mungkin juga menyukai
- ChemistryDokumen2 halamanChemistryVita Faridiana100% (1)
- Chemistry Reviewer 1Dokumen13 halamanChemistry Reviewer 1John Van Dave TaturoBelum ada peringkat
- Advanced Pharmaceutical analysisDari EverandAdvanced Pharmaceutical analysisPenilaian: 4.5 dari 5 bintang4.5/5 (2)
- Ether: Navigation Search AetherDokumen7 halamanEther: Navigation Search AetherMuhammad Wahyu Nugraha0% (1)
- Tabel PeriodikDokumen1 halamanTabel Periodikkuda_tembakBelum ada peringkat
- Structure and Synthesis of Alcohols: Organic Chemistry, 7Dokumen52 halamanStructure and Synthesis of Alcohols: Organic Chemistry, 7haha_le12100% (1)
- Electrical Installation GuideDokumen15 halamanElectrical Installation GuideVanna Rebekah IbatuanBelum ada peringkat
- Histology of Plant and Animal CellsDokumen4 halamanHistology of Plant and Animal Cellscikaifa40% (5)
- Centinela Site Visit Presentation Dec 5 2016Dokumen31 halamanCentinela Site Visit Presentation Dec 5 2016Pili Torres OrregoBelum ada peringkat
- Thermite and IceDokumen3 halamanThermite and IceAlissa GayBelum ada peringkat
- Organic Chemistry,: Alcohols, Ethers, EpoxidesDokumen69 halamanOrganic Chemistry,: Alcohols, Ethers, EpoxidesilhamfaturachmanagusBelum ada peringkat
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersDari EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersBelum ada peringkat
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDari EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsBelum ada peringkat
- ASTM - D6386-99 (Reapproved 2005)Dokumen4 halamanASTM - D6386-99 (Reapproved 2005)tkBelum ada peringkat
- Unit 11 Alcohols Ethers Thiols UST Template 1Dokumen31 halamanUnit 11 Alcohols Ethers Thiols UST Template 1Daniel BalubalBelum ada peringkat
- Ether and AldehydeDokumen112 halamanEther and Aldehydejhapindra adhikariBelum ada peringkat
- Chap IV - Etherepox - ThiolsulfidesDokumen36 halamanChap IV - Etherepox - Thiolsulfideshelia.jafari2Belum ada peringkat
- EtherDokumen23 halamanEtherOng Kok LeongBelum ada peringkat
- Organic Reactions PPT Alcohols.pptDokumen43 halamanOrganic Reactions PPT Alcohols.pptsmithsashay74Belum ada peringkat
- DENT1002-Lecture18-Ethers Epoxides and ThiolsDokumen32 halamanDENT1002-Lecture18-Ethers Epoxides and Thiolszlarsson127Belum ada peringkat
- Alcohol Ether and ExpoksideDokumen64 halamanAlcohol Ether and ExpoksideAhmadBadruzzamanShuib100% (1)
- Chapter Four PowerpointDokumen109 halamanChapter Four PowerpointthanaBelum ada peringkat
- Alcohols, Phenols and EpoxidesDokumen134 halamanAlcohols, Phenols and EpoxidesStudent 365100% (1)
- Alcohols, Phenols and Ethers: Functional Groups and ReactionsDokumen74 halamanAlcohols, Phenols and Ethers: Functional Groups and ReactionsSmit PatelBelum ada peringkat
- Alcohols ClassDokumen29 halamanAlcohols ClassRyan JamesBelum ada peringkat
- The Alchemy of Alcohols:: A Beginner's Guide in Organic ChemistryDokumen17 halamanThe Alchemy of Alcohols:: A Beginner's Guide in Organic ChemistrypentojochaunceyBelum ada peringkat
- Ethers and Epoxides Thiols and SulfidesDokumen37 halamanEthers and Epoxides Thiols and SulfidessarahBelum ada peringkat
- 7_Ethers and EpoxidesDokumen37 halaman7_Ethers and Epoxidesrna6802Belum ada peringkat
- Reactions of AlcoholsDokumen3 halamanReactions of AlcoholsKimBelum ada peringkat
- Alkohol, Eter Dan EpoksidaDokumen72 halamanAlkohol, Eter Dan EpoksidaAdi Kurniawan EffendiBelum ada peringkat
- Alcohols 1Dokumen13 halamanAlcohols 1Suresh VedpathakBelum ada peringkat
- Understanding Alcohols: Properties, Classification and UsesDokumen11 halamanUnderstanding Alcohols: Properties, Classification and UsesSiddhant PatelBelum ada peringkat
- Alcohols, Phenols & EthersDokumen27 halamanAlcohols, Phenols & Ethershgp9ms5gjcBelum ada peringkat
- Organic Compounds Containing OxygenDokumen73 halamanOrganic Compounds Containing OxygenGepsa AprilianaBelum ada peringkat
- Alcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsDokumen12 halamanAlcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsAmihanBelum ada peringkat
- Hydroxy compoundDokumen37 halamanHydroxy compoundZuhailimuna MudaBelum ada peringkat
- Alcohols LabDokumen7 halamanAlcohols Lab7sky7harveyBelum ada peringkat
- Preparation and Reactions of EthersDokumen54 halamanPreparation and Reactions of EthersEj AgsaldaBelum ada peringkat
- Carboxylic Acid Derivatives and Their ReactionsDokumen8 halamanCarboxylic Acid Derivatives and Their ReactionsBoobalan Maria SusaiBelum ada peringkat
- Lectures 19-22 (LB) Alcohols-Phenols-EthersDokumen61 halamanLectures 19-22 (LB) Alcohols-Phenols-Ethersvintu pvBelum ada peringkat
- Alcohols: K. Atkins IB Chemistry Pensacola High SchoolDokumen16 halamanAlcohols: K. Atkins IB Chemistry Pensacola High SchoollatestuserBelum ada peringkat
- CHM 102 - Alcohols-2Dokumen7 halamanCHM 102 - Alcohols-2Philip OkunoyeBelum ada peringkat
- Ethers: Naming, Preparation and PropertiesDokumen10 halamanEthers: Naming, Preparation and PropertiesSaima NajmBelum ada peringkat
- Module 4 - Alcohol, Ether and AldehydeDokumen62 halamanModule 4 - Alcohol, Ether and AldehydePrincess NavarroBelum ada peringkat
- Chem Notes No6Dokumen31 halamanChem Notes No6AnyhaBelum ada peringkat
- EthersDokumen16 halamanEthersajay gudlaBelum ada peringkat
- EthersDokumen16 halamanEthersPriskoBelum ada peringkat
- Chapter 2.4 Alcohol, Ether & EpoxidesDokumen52 halamanChapter 2.4 Alcohol, Ether & Epoxides0JTINGBelum ada peringkat
- AgentsDokumen21 halamanAgentsmeomeogaugau26Belum ada peringkat
- Organic Chemistry PPT - Ch. 22 - 11th Grade - II TERM - DeC'23Dokumen138 halamanOrganic Chemistry PPT - Ch. 22 - 11th Grade - II TERM - DeC'23Ana Pamela MejiaBelum ada peringkat
- KD 2 (Pertemuan 5-6) PDFDokumen93 halamanKD 2 (Pertemuan 5-6) PDFMaulana SaktiBelum ada peringkat
- Introduction To Ethers James Richard FrommDokumen12 halamanIntroduction To Ethers James Richard FrommFrician Bernadette MuycoBelum ada peringkat
- Physical and Chemical Properties of AlcoholsDokumen24 halamanPhysical and Chemical Properties of AlcoholsmeerasahibfarhanBelum ada peringkat
- 307 AlkanesDokumen45 halaman307 AlkanesFAHEEM UD DINBelum ada peringkat
- 4B Reactions of Aldehydes and KetonesDokumen15 halaman4B Reactions of Aldehydes and KetonesAnloraine GonzalesBelum ada peringkat
- Alcohols, Phenols and EthersDokumen51 halamanAlcohols, Phenols and EthersFatehBelum ada peringkat
- Name - Shivpoojan Singh Course-B.Sc (Hon.) Maths ROLL NO - 202110203110008Dokumen17 halamanName - Shivpoojan Singh Course-B.Sc (Hon.) Maths ROLL NO - 202110203110008Siddhant PatelBelum ada peringkat
- Ethers and Epoxides: Ethers Nomenclature of EthersDokumen10 halamanEthers and Epoxides: Ethers Nomenclature of EtherssarahBelum ada peringkat
- ALDEHYSES (Autosaved)Dokumen46 halamanALDEHYSES (Autosaved)az713511Belum ada peringkat
- Ethers and Epoxides Thiols and SulfidesDokumen18 halamanEthers and Epoxides Thiols and SulfidesTrescia Mae EstilloreBelum ada peringkat
- SKO3013 Aldehyde Ketone-Student NoteDokumen51 halamanSKO3013 Aldehyde Ketone-Student NoteMike EzioBelum ada peringkat
- Organic Presentation: Maam Sophia AwaisDokumen30 halamanOrganic Presentation: Maam Sophia AwaisAMMAR AHMEDBelum ada peringkat
- Notes CH 14 Wade 7Dokumen14 halamanNotes CH 14 Wade 7tonyromofan100% (1)
- Chapter 7 AlcoholsDokumen94 halamanChapter 7 Alcoholspammi.radhakrishna.0743Belum ada peringkat
- AlcoholDokumen78 halamanAlcoholMike EzioBelum ada peringkat
- Organic Chemistry: Structure and Synthesis of AlcoholsDokumen58 halamanOrganic Chemistry: Structure and Synthesis of AlcoholsYahya IsiedBelum ada peringkat
- Lecture 26. Aldehyde Presentation by Group 7Dokumen27 halamanLecture 26. Aldehyde Presentation by Group 7Ali RazaBelum ada peringkat
- Alcoholes 1Dokumen36 halamanAlcoholes 1Alan cortes lopezBelum ada peringkat
- 3 11 RPH t4 Label StrukturDokumen1 halaman3 11 RPH t4 Label StrukturcikaifaBelum ada peringkat
- Twinkle Twinkle Little Star Nursery RhymeDokumen3 halamanTwinkle Twinkle Little Star Nursery RhymecikaifaBelum ada peringkat
- Invitation CardDokumen1 halamanInvitation CardcikaifaBelum ada peringkat
- Soalan Pecutan GC Set 4Dokumen25 halamanSoalan Pecutan GC Set 4cikaifaBelum ada peringkat
- Sistem Sticker Good JobDokumen2 halamanSistem Sticker Good JobcikaifaBelum ada peringkat
- SkeemaDokumen3 halamanSkeemaAnaz Freshkin WhiteBelum ada peringkat
- Bookmarks 100Dokumen20 halamanBookmarks 100cikaifaBelum ada peringkat
- Nombor BekasDokumen1 halamanNombor BekascikaifaBelum ada peringkat
- Thumbs UpDokumen3 halamanThumbs UpcikaifaBelum ada peringkat
- Twinkle Twinkle Little Star Nursery RhymeDokumen3 halamanTwinkle Twinkle Little Star Nursery RhymecikaifaBelum ada peringkat
- Soalan Pecutan GC Set 4 SkemaDokumen2 halamanSoalan Pecutan GC Set 4 SkemacikaifaBelum ada peringkat
- Nombor BekasDokumen1 halamanNombor BekascikaifaBelum ada peringkat
- Go 6 Soap Making Processes SambunganDokumen3 halamanGo 6 Soap Making Processes SambungancikaifaBelum ada peringkat
- Soalan TMK 3 N 4Dokumen5 halamanSoalan TMK 3 N 4cikaifaBelum ada peringkat
- Go 2 AlloyDokumen7 halamanGo 2 AlloycikaifaBelum ada peringkat
- GO 4 Preparation of Carboxylic AcidDokumen18 halamanGO 4 Preparation of Carboxylic AcidcikaifaBelum ada peringkat
- Go 1 FuelDokumen21 halamanGo 1 FuelcikaifaBelum ada peringkat
- Go 5 Ammonia, Sulphuric Acid, Nitric AcidDokumen21 halamanGo 5 Ammonia, Sulphuric Acid, Nitric AcidcikaifaBelum ada peringkat
- Program Big Semester 1 PismpDokumen20 halamanProgram Big Semester 1 PismpHady Al FarouqBelum ada peringkat
- History of AtomDokumen36 halamanHistory of AtomcikaifaBelum ada peringkat
- Gaseous Exchange in Human and Plant and The Circulatory SystemDokumen26 halamanGaseous Exchange in Human and Plant and The Circulatory Systemcikaifa100% (1)
- Reproduction, Development and GrowthDokumen57 halamanReproduction, Development and GrowthcikaifaBelum ada peringkat
- Kinematics and DynamicsDokumen7 halamanKinematics and DynamicscikaifaBelum ada peringkat
- What Is Science?Dokumen7 halamanWhat Is Science?cikaifaBelum ada peringkat
- Error EstimationDokumen8 halamanError EstimationcikaifaBelum ada peringkat
- Asgment Math Sem 1 PpismpDokumen23 halamanAsgment Math Sem 1 Ppismpcikaifa100% (1)
- Nervous Systen and HormoneDokumen29 halamanNervous Systen and HormonecikaifaBelum ada peringkat
- 2020 GADSL Reference List ProdDokumen220 halaman2020 GADSL Reference List ProdParom WaikasikarnBelum ada peringkat
- Assignment 2 - BUS 336Dokumen3 halamanAssignment 2 - BUS 336Omar Al-lheebiBelum ada peringkat
- Calculation of Thermodynamic Properties of Hydrated BoratesDokumen5 halamanCalculation of Thermodynamic Properties of Hydrated BoratesKary ShitoBelum ada peringkat
- AISI 1018 Carbon Steel Properties and ApplicationsDokumen3 halamanAISI 1018 Carbon Steel Properties and ApplicationsNaman TanejaBelum ada peringkat
- PharChem Acid and Bases PDFDokumen43 halamanPharChem Acid and Bases PDFTinni TapawanBelum ada peringkat
- ME8352-Manufacturing Technology - I (MT-I) With QBDokumen91 halamanME8352-Manufacturing Technology - I (MT-I) With QBMohana KrishnanBelum ada peringkat
- 용접의 개요Dokumen26 halaman용접의 개요박제영Belum ada peringkat
- Zinc PlatingDokumen3 halamanZinc Platingjavier.garcia6281Belum ada peringkat
- Solubility Rules - Chemistry LibreTextsDokumen2 halamanSolubility Rules - Chemistry LibreTextsKonoka KonoeBelum ada peringkat
- Minerals - SilicatesDokumen2 halamanMinerals - SilicateshBelum ada peringkat
- AFA Glossary of TermsDokumen59 halamanAFA Glossary of TermsKe HalimunBelum ada peringkat
- Teóricas - 11. Co-Cr Alloys PDFDokumen14 halamanTeóricas - 11. Co-Cr Alloys PDFrostcarBelum ada peringkat
- The Determination of Antimony, Tin and LeadDokumen7 halamanThe Determination of Antimony, Tin and LeadSoledad ColmenarezBelum ada peringkat
- Nickel, gold, copper and other metals comparedDokumen5 halamanNickel, gold, copper and other metals comparedRofilR.AlbaoBelum ada peringkat
- Chapter 10 The S-Block ElementsDokumen18 halamanChapter 10 The S-Block ElementsYash PlayBelum ada peringkat
- MBH Catalogue 2019Dokumen56 halamanMBH Catalogue 2019Anonymous 1oWzM3Belum ada peringkat
- INORGDokumen3 halamanINORGShinBelum ada peringkat
- Iec 209Dokumen14 halamanIec 209AnamulKabir100% (2)
- 2016 MATERIALOGRAPHIC CONSUMABLES CATALOGUEDokumen68 halaman2016 MATERIALOGRAPHIC CONSUMABLES CATALOGUEGunasekaran paramasamyBelum ada peringkat
- Vanadium and Vanadium CompoundsDokumen21 halamanVanadium and Vanadium CompoundsПлейнBelum ada peringkat
- 2B-Alcohols, Ethers & Phenols - FINAL - 06!03!14 (86-112)Dokumen27 halaman2B-Alcohols, Ethers & Phenols - FINAL - 06!03!14 (86-112)udaysrinivasBelum ada peringkat
- AndlettheslaysloutDokumen100 halamanAndlettheslaysloutsadiyamoha09Belum ada peringkat
- Assignment 2Dokumen3 halamanAssignment 2samy.anesuBelum ada peringkat