Year 12 Chemistry Assessment Task Blakehurst
Diunggah oleh
Lu FangDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Year 12 Chemistry Assessment Task Blakehurst
Diunggah oleh
Lu FangHak Cipta:
Format Tersedia
Year 12 chemistry assessment task When: week 2/3, term 1,2011.
Weighting: 20% What: written assessment task in class Task: prior to the above date, research topic 9.2.5 from the syllabus as listed below. Your assessment task will require you to answer a series of questions based on the dot points, using the information you have researched. You will need to bring your information to the task. Information: can consist of textbooks, other books, class notes, pages from internet, journal articles and written work. YOU need at least 3 sources. All sources except books will be handed in with your completed task. Marks: 25 marks Instructions: in your answers, include all relevant formulae and reactions. USE your own wording and sentences. Refer to the board of studies regulations about plagiarism: all my own work. a bibliography is required. Assessment criteria: these will be attached to your assessment task. Use them to guide your responses to the questions. Syllabus dot pts: 9.2.5. Nuclear chemistry provides a range of materials Students learn to: Distinguish between stable and radioactive isotopes and describe the conditions under which a nucleus is unstable
An element has a specific number of protons and neutrons, if the number of protons changes then the element changes. However if the number of neutrons changes then it is referred to as an isotope. NOT all isotopes are radioactive, the ones that are depend on the proton: neutron ratio For small elements n<20 if the proton: neutron ratio is 1:1 then it is considered STABLE For larger elements n>20 but smaller than n=83 then the proton: neutron ratio is 1:1.5 and it is considered stable ALL elements/isotopes above atomic number 83 are radioactive even though 83-92 are found naturally. Basically being radioactive just means that the nucleus has too many neutrons and it cant stay that way so it decays to a more stable version by emitting alpha, beta particles sometimes accompanied by gamma rays. You can also mention the zone of stabllity, which is a graph of proton: neutron (we did in class)
Describe how transuranic elements are produced
Transuranic elements are all elements above atomic number 92 (uranium), these elements are artificially/synthetically produced in 1 of 2 ways. 1. Cyclotron/particle accelerator 2. Nuclear reactor Please find how these machines BASICALLy work, made 2 sentences for each Describe how commercial radioisotopes are produced
Not all radioisotopes are useful, for instance the ones above atomic number 104 have an extremely short half life therefore not useful in any scientific field. But a commercial radioisotope refers to the ones that have an application/useful and these are produced in either a cyclotron/particle accelerator or nuclear reactor Find an example of one and remember the equn of how its produced Identify instruments and processes that can be used to detect radiation
1. Geiger Muller counter 2. Scintillation counter 3. Cloud chamber Photographic paper can be used to detect the presence of radiation but NOT AN instrument that can be used to measure radiation like the other 3 Use your textbook or internet to write a brief paragraph on the 3 instruments above
Identify one use of a named radioisotope: -in industry -in medicine I suggest you choose technetium-99m (med) and cobalt-60(industry) but you can do others if you choose to Describe the way in which the above named industrial and medical radioisotopes are used and explain their use in terms of their chemical properties
Research and get back to me Students: Process information from secondary sources to describe recent discoveries of elements
On the 9th of November 1994 at 4:39 pm the first atom of the heaviest chemical element with atomic number 110 was detected at the Gesellschaft fr Schwerionenforschung (GSI) in Darmstadt, Germany. Unlike these lighter atoms, element 110 decays after a small fraction of a thousandth of a second into lighter elements by emitting alpha-particles which are the nuclei of helium atoms. The new element was produced by fusing a nickel and a lead atom together. This was achieved by accelerating the nickel atoms to a high energy in the heavy ion accelerator UNILAC at GSI. This rare reaction occurs only at a very specific velocity of the nickel projectile. Over a period of many days, many billion billion nickel atoms must be shot at a lead target in order to produce and identify a single atom of element 110. Element 110 known as darmstadtium basically it was produced in a particle accelerator by the fusion of nickel and lead. Use available evidence to analyse benefits and problems associated with the use of radioactive isotopes in identified industries and medicine
Research and get back to me
Anda mungkin juga menyukai
- Radiopharmaceutics Presentation1Dokumen52 halamanRadiopharmaceutics Presentation1alibinaminBelum ada peringkat
- Tristan TateDokumen3 halamanTristan Tatecelebritydecks1Belum ada peringkat
- Fossils (DK Smithsonian Handbook) by DKDokumen320 halamanFossils (DK Smithsonian Handbook) by DKAnthony Mello71% (7)
- Mushrooms Russia and HistoryDokumen579 halamanMushrooms Russia and Historyzeevnaomi100% (4)
- Chapter 22 Nuclear Chem Study GuideDokumen5 halamanChapter 22 Nuclear Chem Study GuideVicky100% (2)
- Composition Symbol: Gamma Beta AlphaDokumen8 halamanComposition Symbol: Gamma Beta AlphaNatasha EdirisinghegeBelum ada peringkat
- Assignment 1 - Chemistry of The Periodic TableDokumen6 halamanAssignment 1 - Chemistry of The Periodic TableGraceBelum ada peringkat
- Lecture - Notes - Chemistry - Final - 10232018 PDFDokumen130 halamanLecture - Notes - Chemistry - Final - 10232018 PDFAlvin DeliroBelum ada peringkat
- Radio PharmaceuticsDokumen52 halamanRadio PharmaceuticsDrGaurav TiwariBelum ada peringkat
- Chemical Vs Nuclear ReactionsDokumen47 halamanChemical Vs Nuclear Reactionsapi-236069914Belum ada peringkat
- RSO Book - Technology ExpertsDokumen141 halamanRSO Book - Technology ExpertsNaresh KumarBelum ada peringkat
- Presentation NuclearDokumen47 halamanPresentation NuclearMaheshBelum ada peringkat
- Radiation SafetyDokumen67 halamanRadiation SafetyAmit SinghBelum ada peringkat
- Principles and Applications of Liquid Scintillation CountingDokumen15 halamanPrinciples and Applications of Liquid Scintillation CountingDavid BrownBelum ada peringkat
- 2ND Term S3 ChemistryDokumen28 halaman2ND Term S3 ChemistryRhemaBelum ada peringkat
- Title: Quantization of Energy LabDokumen4 halamanTitle: Quantization of Energy Laband thenBelum ada peringkat
- WEEK 5 CDokumen13 halamanWEEK 5 CeodurotwumBelum ada peringkat
- Radiation Safety PDFDokumen67 halamanRadiation Safety PDFHoàng Việt AnhBelum ada peringkat
- Flame Test Lab Wire LoopsDokumen6 halamanFlame Test Lab Wire LoopsEjay OlebogengBelum ada peringkat
- 10.1 Understanding The Nucleus of An Atom 10.1.1 Composition of The NucleusDokumen14 halaman10.1 Understanding The Nucleus of An Atom 10.1.1 Composition of The NucleusrajhiniBelum ada peringkat
- Nuclear MedicineDokumen26 halamanNuclear Medicinesherinwidya williamsBelum ada peringkat
- Term Paper About Nuclear ChemistryDokumen7 halamanTerm Paper About Nuclear Chemistrybzknsgvkg100% (1)
- Radiopharmaceuticals 1Dokumen56 halamanRadiopharmaceuticals 1salinaBelum ada peringkat
- Flame Spectroscopy and AasDokumen27 halamanFlame Spectroscopy and Aasgayatri maldhureBelum ada peringkat
- Light Energy: Solar Cell Based On Artificial PhotosynthesisDokumen21 halamanLight Energy: Solar Cell Based On Artificial PhotosynthesisifmatosBelum ada peringkat
- Phy ChemDokumen248 halamanPhy ChemBitter SugarBelum ada peringkat
- Grade 11 - Week 2 - Sept 17 GÇô Sept 21, 2012Dokumen5 halamanGrade 11 - Week 2 - Sept 17 GÇô Sept 21, 2012Jerrord ThomasBelum ada peringkat
- VSI Week9 Lecture9 InstAnal 4th Stage Theory 2022Dokumen34 halamanVSI Week9 Lecture9 InstAnal 4th Stage Theory 2022Sozdar ArgoshiBelum ada peringkat
- Radiation Safety: ἄτομος or átomos meaning "indivisible") is the smallest particle of aDokumen10 halamanRadiation Safety: ἄτομος or átomos meaning "indivisible") is the smallest particle of adypietBelum ada peringkat
- Principles and Applications of Atomic Abso R Pti N Spectroscopy AlfredDokumen62 halamanPrinciples and Applications of Atomic Abso R Pti N Spectroscopy AlfredSazBelum ada peringkat
- 1 - Lab Safety & IR SpectrosDokumen6 halaman1 - Lab Safety & IR Spectrosc88y45hbnqBelum ada peringkat
- MST, Module 1, NotesDokumen22 halamanMST, Module 1, NotesChandrashekhar KulkarniBelum ada peringkat
- CIE Modern Physics Sample PagesDokumen39 halamanCIE Modern Physics Sample PagesrenedavidBelum ada peringkat
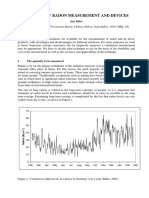
- Methods of Radon Measurement and DevicesDokumen8 halamanMethods of Radon Measurement and DevicesVictor Sender RodríguezBelum ada peringkat
- Summary of Chemistry Textbook - Section 1.1 The AtomDokumen3 halamanSummary of Chemistry Textbook - Section 1.1 The AtomRachel JeffresonBelum ada peringkat
- KPD 3016: Pengajaran Teknologi Dan Penaksiran 1 Kumpulan A: Tugasan 3Dokumen11 halamanKPD 3016: Pengajaran Teknologi Dan Penaksiran 1 Kumpulan A: Tugasan 3SMAF3016Belum ada peringkat
- Chemistry Notes Pt. 1Dokumen108 halamanChemistry Notes Pt. 1weny fidayBelum ada peringkat
- Nuclear Chemistry - ShieldingDokumen2 halamanNuclear Chemistry - ShieldingMichael Larry MAGPARANGALANBelum ada peringkat
- Section 1.4: Isotopes, Radioisotopes, and Atomic MassDokumen4 halamanSection 1.4: Isotopes, Radioisotopes, and Atomic MassgzboyzoneBelum ada peringkat
- Chemistry Nuclear Chem NotesDokumen18 halamanChemistry Nuclear Chem Notesmonishsarker564Belum ada peringkat
- What Is Radioactivity Homework 55Dokumen7 halamanWhat Is Radioactivity Homework 55afeuhyxst100% (1)
- STUDENT ChemTourWorkbook2022v01Dokumen22 halamanSTUDENT ChemTourWorkbook2022v01Dhyey PatelBelum ada peringkat
- Research Paper About Nuclear ChemistryDokumen4 halamanResearch Paper About Nuclear Chemistrywhrkeculg100% (1)
- Nuclear EnergyDokumen81 halamanNuclear EnergyEmy AnkrahBelum ada peringkat
- Radioactive DecayDokumen2 halamanRadioactive DecayShah Rafidzah KhalidBelum ada peringkat
- Are View On Nuclear ChemistryDokumen7 halamanAre View On Nuclear ChemistrySehar fatimaBelum ada peringkat
- Chapter - 19 The Atomic NucleusDokumen10 halamanChapter - 19 The Atomic Nucleususama113Belum ada peringkat
- RADIOGRAPHY STUDY MATERIAL LatestDokumen52 halamanRADIOGRAPHY STUDY MATERIAL LatestManish SinghBelum ada peringkat
- (k4) Materi Kuliah RadiobiologiDokumen22 halaman(k4) Materi Kuliah RadiobiologiwidyaBelum ada peringkat
- Chem 113E Module 5 Nuclear ChemistryDokumen20 halamanChem 113E Module 5 Nuclear ChemistryKenneth John FerrarizBelum ada peringkat
- 1962 Fallout Shelter DesignDokumen218 halaman1962 Fallout Shelter DesignLouie_popwhatski100% (1)
- Unit 1Dokumen175 halamanUnit 1Jonny SinglaBelum ada peringkat
- Flame Tests (Lab 1)Dokumen4 halamanFlame Tests (Lab 1)Kaye ReiesBelum ada peringkat
- Topic 7.1 - Discrete Energy and RadioactivityDokumen85 halamanTopic 7.1 - Discrete Energy and RadioactivityPaul Amezquita100% (4)
- AtomicDokumen41 halamanAtomicHassanKMBelum ada peringkat
- College of EngineeringDokumen17 halamanCollege of EngineeringClarkBelum ada peringkat
- RadioactivityDokumen12 halamanRadioactivityAya WalidBelum ada peringkat
- Uses of Radioactivity Including Radioactive DatingDokumen27 halamanUses of Radioactivity Including Radioactive DatingLia CBelum ada peringkat
- 4.7 RadioactivityDokumen14 halaman4.7 Radioactivitygabrielsuva6Belum ada peringkat
- A Translation Quality Assessment of Two English Translations of Nazım HikmetDokumen14 halamanA Translation Quality Assessment of Two English Translations of Nazım HikmetIntzarEltlBelum ada peringkat
- Criminal Law Book 2 Titles 1-8Dokumen146 halamanCriminal Law Book 2 Titles 1-8Minato NamikazeBelum ada peringkat
- 3 - QMT425-T3 Linear Programming (29-74)Dokumen46 halaman3 - QMT425-T3 Linear Programming (29-74)Ashraf RadzaliBelum ada peringkat
- SkripsiDokumen101 halamanSkripsiNurul Maharani PutriBelum ada peringkat
- Delhi Public School, Agra: The HolocaustDokumen3 halamanDelhi Public School, Agra: The Holocaustkrish kanteBelum ada peringkat
- Construction Quality Management 47 PDFDokumen47 halamanConstruction Quality Management 47 PDFCarl WilliamsBelum ada peringkat
- MA in Public Policy-Handbook-2016-17 (KCL)Dokumen14 halamanMA in Public Policy-Handbook-2016-17 (KCL)Hon Wai LaiBelum ada peringkat
- The Creative Bureaucracy 2Dokumen45 halamanThe Creative Bureaucracy 2IndirannaBelum ada peringkat
- Chapter 1 To 3. Methods of ResearchDokumen18 halamanChapter 1 To 3. Methods of ResearchMaryAnnLasquiteBelum ada peringkat
- 6 Money SupplyDokumen6 halaman6 Money SupplySaroj LamichhaneBelum ada peringkat
- TaTa TeA Ad AnaLysiSDokumen3 halamanTaTa TeA Ad AnaLysiSAmiya RautBelum ada peringkat
- MultidisciplinaryDokumen20 halamanMultidisciplinaryrabiaBelum ada peringkat
- University of Mumbai: Bachelor of Management Studies (Finance) Semester VIDokumen73 halamanUniversity of Mumbai: Bachelor of Management Studies (Finance) Semester VIPranay ShettyBelum ada peringkat
- Alumni - 1997Dokumen132 halamanAlumni - 1997GSL Medical CollegeBelum ada peringkat
- What Does Nature MeanDokumen9 halamanWhat Does Nature MeanSo DurstBelum ada peringkat
- Brahm Dutt v. UoiDokumen3 halamanBrahm Dutt v. Uoiswati mohapatraBelum ada peringkat
- Notes On The "Historical Turn" and The Uses of Theory in Film Studies - ARTDokumen7 halamanNotes On The "Historical Turn" and The Uses of Theory in Film Studies - ARTaarmherBelum ada peringkat
- Oils and Lard by Fourier Transform Infrared Spectroscopy. Relationships Between Composition and Frequency of Concrete Bands in The Fingerprint Region.Dokumen6 halamanOils and Lard by Fourier Transform Infrared Spectroscopy. Relationships Between Composition and Frequency of Concrete Bands in The Fingerprint Region.Nong NakaBelum ada peringkat
- Design ManagementDokumen21 halamanDesign ManagementKarishma Mittal100% (1)
- Psychological Science1Dokumen23 halamanPsychological Science1Jatin RaghavBelum ada peringkat
- Quarter 4 Week 4 Parallel Lines Cut by A TransversalDokumen26 halamanQuarter 4 Week 4 Parallel Lines Cut by A TransversalZaldy Roman MendozaBelum ada peringkat
- Muhammad Fa'iz Annur: Daftar Hasil Studi Dan Yudisium (Dhsy)Dokumen1 halamanMuhammad Fa'iz Annur: Daftar Hasil Studi Dan Yudisium (Dhsy)osamafania nadiumaraBelum ada peringkat
- Power Semiconductor DevicesDokumen11 halamanPower Semiconductor DevicesBhavesh Kaushal100% (1)
- Ass HeniDokumen14 halamanAss Henialexsabebe28Belum ada peringkat
- 18.CASE STUDY - Southwest AirlinesDokumen23 halaman18.CASE STUDY - Southwest AirlinesDaniel CiprianBelum ada peringkat
- Degenesis Rebirth Edition - Interview With Marko DjurdjevicDokumen5 halamanDegenesis Rebirth Edition - Interview With Marko DjurdjevicfoxtroutBelum ada peringkat
- Lantus SoloStar Pen Guide PDFDokumen7 halamanLantus SoloStar Pen Guide PDFKai HarukaBelum ada peringkat