Notes
Diunggah oleh
rachel_o_reilly6399Deskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Notes
Diunggah oleh
rachel_o_reilly6399Hak Cipta:
Format Tersedia
Endocrine/Reproductive Physiology
Peptide hormones
These are the most abundant class of hormones (<1000). They are water soluble meaning they are easily transported in the blood. They do not readily cross cell membranes. Instead, they interact with receptors on the cell surface and use second messengers to send intracellular signals. These hormones are synthesized by the rough endoplasmic reticulum to produce the preprohormone. The signal peptides are then removed producing prohormones. They undergoe post translational modification in the golgi apparatus and are packaged into secretory vesicles. These can then be excreted from the cell by exocytosis in response to certain stimuli. They are highly conserved in evolution. Some of the main families include glycoprotein hormones, growth hormone/prolactin family and the parathyroid family. Several important peptide hormones are secreted from the pituitary gland. The anterior pituitary secretes prolactin, which acts on the mammary gland, adrenocorticotrophic hormone (ACTH), which acts on the adrenal cortex to regulate the secretion of glucocorticoids, and growth hormone, which acts on bone, muscle, and the liver. The posterior pituitary gland secretes antidiuretic hormone, also called vasopressin, and oxytocin
Amine hormones
This group of hormones is made up of the catecholamines and the thyroid hormones. Norepinephrine, epinephrine and dopamine are all derived from a single amino acid, tyrosine. Norepinephrine and epinephrine function as both hormones (E>NE) and neurotransmitters (NE>E). They are synthesized by chromaffin cells located in the adrenal medulla. They are water soluble molecules and are easily transported in the blood. They are rapidly metabolised by one or both of the following enzymes; monoamine oxidase and catechol -O- methyltransferase. They are stored in secretory vesicles with the main stimulus for secretion being sympathetic stimulation by the splanchnic nerve. The thyroid hormones, triiodothyronine (T3) and thyroxine (T4) are synthesised in thyroid follicular cells. Thyroglobulin is digested by lysosomal enzymes into the cell followed by iodination of the tyrosine residues. They are stored in large extracellular deposits known as thyroid follicles. T3 and T4 can then be released with the iodine being recycled. This system is regulated primarily by TSH. There is approximately 5mg in the average adult gland with a turnover of about 1% per day. They are relatively non polar and have limited solubility in water. They can diffuse across the plasma membrane where they target intracellular/nuclear receptors. They require plasma transport proteins to reach these target cells.
Steroid hormones
All steroid hormones are derived from cholesterol. They can be grouped into five groups by the receptors to which they bind: glucocorticoids, mineralocorticoids, androgens, estrogens and progestogens. They are generally synthesized from cholesterol in the gonads and adrenal
glands. These forms of hormones are lipids. They can pass through the cell membrane as they are fat-soluble, and then bind to steroid hormone receptors which may be nuclear or cytosolic depending on the steroid hormone, to bring about changes within the cell. They are not stored but are immediately released from the cell following synthesis. Steroid hormones are generally carried in the blood bound to specific carrier proteins such as sex hormone-binding globulin or corticosteroidbinding globulin. Further conversions and catabolism occurs in the liver, in other "peripheral" tissues, and in the target tissues. They are slower acting and have a longer half-life than the peptide hormones.
Hormone receptors
A hormone receptor is a molecule that can bind to a specific hormone. Upon hormone binding, the receptor can initiate multiple signalling pathways which ultimately lead to changes in the behaviour of the target cells. Binding affinity of receptor is defined by an equilibrium constant called the Kd, or dissociation constant. The lower the Kd, the greater the hormone binding affinity. The binding affinity is defined as the concentration of the hormone at which 50% of receptors are occupied. Sensitivity is defined as hormone concentration giving half maximal response. Hormone sensitivity does not always parallel hormone binding or receptor affinity. The greater the proportion of spare receptors, the more sensitive the target cell is to the hormone. Receptors for peptide hormones tend to be found on the plasma membrane of cells, whereas receptors for lipid-soluble hormones are usually found within the cytoplasm. These cell membrane receptors include ion channel linked, G protein coupled and enzyme linked receptors. G protein coupled receptors are transmembrane receptors made up of seven domains. The extracellular parts of the receptor can be glycosylated. These extracellular loops also contain two highly-conserved cysteine residues that form disulfide bonds to stabilize the receptor structure. Binding of a hormone induces a conformational change in the receptor. The activated receptor then binds to a G subunit triggering a conformational change and triggering dissociation of GDP. Binding of GTP to G triggers dissociation of G both from the receptor and from G. The hormone dissociates from the receptor and G binds to the effector activating it. Hydrolysis of GTP to GDP causes G to dissociate from the effector and reassociate with G.
Second messengers
Second messengers are molecules that relay signals from receptors on the cell surface to target molecules inside the cell, in the cytoplasm or nucleus. They can be small diffusible (water or lipid soluble) molecules such as cAMP, Ca2+ and phospholipids (inositol 3 phosphate (IP3) anddiacylglycerol (DAG)). They are responsible for the activation of protein kinase A and protein kinase C. The second messengers that are produced as a result of G protein activation serve to regulate the activity of cellular protein kinases, such as the activation of cAMP-dependent protein kinase (protein kinase A [PKA]) and PKC by cAMP and calcium/phospholipids respectively.
Assessment of endocrine function
Bioassays can measure hormone activity in a living system. They have the advantage of being able to measure activity and not hormonal levels, however, take a long time and lack precision and sensitivity so are not practical for clinical use. Radioimmunoassay (IRA) is a competitive binding assay that uses radio-labelled hormone and a single antibody prepared against the specific hormone as a binding site. Competition between unlabelled hormone in the patient sample and the addedlabelled hormone for a limited number of antibody-binding sites forms the basis of the assay. Immunoradiometric assay (IRMA) is similar to RIA in that a radiolabeled substance is used in an antibody-antigen reaction. However, the radioactive label is attached to an antibody instead of the hormone Immunometric assays involve immobilizing antibodies onto a plastic surface (most often a 96-well microtiter plate) to capture the target antigen present in the sample. A second antibody linked to an enzyme (the conjugate) is then added. It binds to a different location on the target antigen. Plate wells are washed to remove unbound components. Substrate is added. The bound enzyme present reacts with the substrate, yielding colour. The amount of hormone present is directly proportional to the amount of radioactivity/absorbance measured in the assay (not displacement method).
Hypothalamus and posterior pituitary
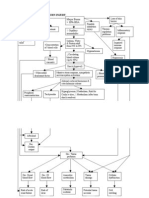
One of the most important functions of the hypothalamus is to link the nervous system to the endocrine system via the pituitary gland (hypophysis). It plays a vital role in maintaining homeostasis and other activities of the autonomic nervous system. As a limbic system structure, the hypothalamus also influences various emotional responses. It is located on either side of the 3rd ventricle and contains a number of nuclei involved in neuroendocrine control; the paraventricular nucleus, the supraoptic nucleus, the ventromedial nucleus, the arcuate nucleus and the periventricular zone. The hypothalamus is responsible for the release of the hypophysiotropic hormones into the anterior pituitary. These include gonadotropin-releasing hormone (GnRH), growth hormone-releasing hormone (GHRH), growth hormone inhibitory hormone (somatostatin), thyrotropin-releasing hormone (TRH), prolactin-inhibiting hormone (PIH) and corticotropin-releasing hormone (CRH). Cortisol secretion is one of the principal components of the endorcrine response to stress. It acts on liver and skeletal muscle to mobilise glucose/energy source. CRH is secreted by the paraventricular nucleus of the hypothalamus in response to stress. This results in the increased secretion of Adrenocorticotropic hormone (ACTH) in the anterior pituitary. The efferent output from the PVN also activates the sympathetic branch of the autonomic system releasing epinephrine. The hypothalamus senses low circulating levels of thyroid hormone (T3 and T4) and responds by releasing thyrotropin-releasing hormone (TRH) from the neuroendocrine cells in the paraventricular nucleus. The TRH stimulates the pituitary to produce thyroid-stimulating hormone (TSH). The TSH, in turn, stimulates the thyroid to produce thyroid hormone until levels in the blood return to normal. Thyroid hormone exerts negative feedback control over the hypothalamus as well as anterior pituitary, thus controlling the release of both TRH from hypothalamus and TSH from anterior pituitary gland.
This axis controls development, reproduction, and aging in animals. The hypothalamus is located in the brain and secretes GnRH. GnRH travels down the anterior portion of the pituitary via the hypophyseal portal system and binds to receptors on the secretory cells of the adenohypophysis. In response to GnRH stimulation these cells produce LH and FSH, which travel into the blood stream. These two hormones play an important role in communicating to the gonads. In females, LH acts primarily to activate the ovaries to produce estrogen via theca cells and FSH causes synthesis of inhibin via granulosa cells regulating the menstrual cycle and ovarian cycle. Estrogen forms a negative feedback loop by inhibiting the production of GnRH in the hypothalamus. Inhibin acts to inhibit activin, which is a peripherally produced hormone that positively stimulates GnRH producing cells. Follistatin which is also produced in all body tissue, inhibits activin and gives the rest of the body more control over the axis. In males LH stimulates leydig cells located in the testes to produce testosterone, and FSH plays a role in spermatogenesis by acting on sertoli cells. Only small amounts of estrogen are secreted in males. Recent research has shown a neurosteroid axis exists, which help the cortex to regulate the hypothalamuss production of GnRH. Genetic mutations and chromosomal abnormalities are two sources of HPG axis alteration. Single mutations usually lead to changes in binding ability of the hormone and receptor leading to inactivation or over activation. These mutations can occur in the genes coding for GnRH, LH, and FSH or their receptors For example, in males mutations in the GnRH coding gene could result in hypogonadotrophic hypogonadism.
Posterior pituitary
Water retention is a necessary process controlled by antidiuretic hormone (ADH), or arginine vasopressin (AVP) as it is also known. It is synthesized by the magnocellular neurons of the supraoptic and paraventricular nuclei located in the hypothalamus. The preprohormone undergoes transformation to it active form while it is being transferred to the stalk of the posterior pituitary. It is retained in the axonal projections of the neurohypophysis, or posterior pituitary, and released from the nerve endings into the bloodstream in response to certain stimuli. It acts on the collecting tubules located in the kidneys causing reabsorption of water and increased urine concentration. ADH is tonically secreted into our bloodstream. The regulation of this hormone is controlled by two systems; plasma osmolality and blood pressure and volume. This fine-tuned electrolyte-water balance system uses osmoreceptors which can sense the slightest change in osmolality, causing the increased release of ADH into the bloodstream when a state of diuresis is necessary(Arima et al., 1998). Diabetes insipidus arises when there is problem with ADH production/secretion or when the kidneys become unresponsive to the hormone. Large amounts of dilute, hypotonic urine are eliminated despite the risk of dangerous dehydration. See essay. Oxytocin is structurally similar to vasopressin. The major site of OT gene expression is the magnocellular neurons of the hypothalamic paraventricular and supraoptic nuclei. In response to a variety of stimuli such as suckling, parturition, or certain kinds of stress, the processed OT peptide is released from the posterior pituitary into the systemic circulation. Such stimuli also lead to an intranuclear release of OT. Moreover, oxytocinergic neurons display widespread projections throughout the central nervous system. However, OT is also synthesized in peripheral tissues, e.g., uterus, placenta, amnion, corpus luteum, testis, and heart. The OT receptor is a typical class I G
protein-coupled receptor that is primarily coupled via Gq proteins to phospholipase C-. The highaffinity receptor state requires both Mg2+ and cholesterol, which probably function as allosteric modulators. The classical actions of OT are stimulation of uterine smooth muscle contraction during labor and milk ejection during lactation. The central actions of OT range from the modulation of the neuroendocrine reflexes to the establishment of complex social and bonding behaviors related to the reproduction and care of the offspring.
Anterior pituitary
The anterior pituitary, or adenohypophysis, is regulated by three levels of controls. Hypothalamic control is mediated by adenohypophysiotropic hormones secreted into the portal system and impinging directly upon anterior pituitary cell surface receptors. Peripheral hormones also participate in mediating pituitary cell function, predominately by negative feedback regulation of trophic hormones by their respective target hormones. Intrapituitary paracrine and autocrine soluble growth factors and cytokines act locally to regulate neighbouring cell development and function. The net result of these three tiers of complex intracellular signals is the controlled pulsatile secretion of the six pituitary trophic hormones, ACTH, GH, PRL, TSH, FSH and LH, through the cavernous sinus, petrosal veins, and ultimately the systemic circulation through the superior vena cava. Five distinct hormone-secreting cell types are present in the mature anterior pituitary gland; Corticotrophs (ACHT), gonadotrophs (FSH and LH), thyrotrophs (TSH), lactotrophs (prolactin) and somatotrophs (GH). Rathkes pouch, a primitive ectodermal invagination anterior to the roof of the oral cavity, is formed by the fourth to fifth week of gestation and gives rise to the anterior pituitary gland.
Prolactin
Prolactin is the only major anterior pituitary hormone that is not a trophic hormone. Its secretion is under the inhibitory control of dopamine, which is largely produced by the tuberoinfundibular (TIDA) cells, and the hypothalamic tuberohypophyseal dopaminergic system. DA reaches the lactotrophs through the hypothalamic pituitary portal system and inhibits PRL secretion by binding to D2 receptors on pituitary lactotrophs. PRL in turn, participates in negative feedback to control its release by increasing tyrosine hydroxylase activity in the TIDA neurons. Factors others than DA inhibit PRL secretion including endothelin-1, transforming growth factor B1 and calcitonin. Estrogen, TRH and basic epidermal growth factor increase PRL synthesis and secretion. The PRL receptor gene is a member of the cytokine receptor superfamily. The PRL receptor induces protein tyrosine phosphorylation and activation of JAK2 kinase and STATS 1 to 5. PRL receptors are expressed in breast, pituitary, liver, adrenal cortex, kidneys, prostate, ovary, testes, intestine, epidermis, pancreatic islets, lung, myocardium, brain and lymphocytes. PRL is essential for human survival because of its role in milk production during pregnancy and lactation.
Growth Hormone
Somatotrophs are located predominantly in the lateral wings of the anterior pituitary gland and constitute 35-45% of pituitary cells. The GH molecule is synthesized, stored and secreted by somatotroph cells. GH is regulated by its target growth factor, IGF-1 which participates in a
hypothalamic-pituitary peripheral regulatory feedback system. GH stimulates IGF-1 which exerts a negative feedback effect on the hypothalamus and pituitary. GH acts to mediate growth and metabolic functions. It elicits intracellular signalling through a peripheral receptor and initiates a phosphorylation cascade involving the JAK/STAT pathway. It regularly pulses at 2-3hr intervals with peak secretion coinciding with onset of deep sleep. The liver contains abundant GH receptors and several peripheral tissues also express modest amounts of receptor, including muscle and fat. GH binding proteins function to damp acute oscillations in serum GH levels associated with pulsatile pituitary GH secretion, and the plasma half-life of GH is prolonged by decreased renal clearance of bound GH. The high-affinity BP also prevents GH binding to surface GH receptors by competing for the GH ligand. GH resistance, as demonstrated in malnutrition, chronic liver disease, short stature, Laron dwarfism, and some African pygmies, is characterised by decreased BP levels in plasma. Acromegaly is a syndrome that results when the anterior pituitary gland produces excess growth hormone (GH) after epiphyseal plate closure at puberty. GH continues to be secreted in adulthood after growth cessation, implying important metabolic functions of GH in adult life. GH increases fat mobilization, decreases fat deposition, and activates hormone-sensitive lipase, resulting in increased triglyceride hydrolysis to free fatty acids and glycerol (lipolysis) and also decreased fatty acid reesterification. As GH is degraded in the kidney, GH levels are elevated in patients with chronic renal failure.
Adrenocorticotropic hormone
Corticotroph cells constitute about 20% of functional anterior pituitary cells. POMC is the precursor of ACTH which acts on the adrenal glands to induce synthesis and secretion of adrenal steroids. CRH is e released from neurosecretory nerve terminals at the median eminence. CRH is transported to the anterior pituitary through the portal blood vessel system of the hypophyseal stalk. There, CRH and vasopressin act synergistically to stimulate the secretion of stored ACTH from corticotrope cells. ACTH is transported by the blood to the adrenal cortex of the adrenal gland, where it rapidly stimulates biosynthesis of corticosteroids such as cortisol from cholesterol. Cortisol is a major stress hormone and has effects on many tissues in the body, including on the brain. In the brain, cortisol acts at two types of receptor - mineralocorticoid receptors and glucocorticoid receptors, and these are expressed by many different types of neurons. One important target of glucocorticoids is the hypothalamus, which is a major controlling centre of the HPA axis.
Thyroid stimulating hormone
Thyrotroph cells constitute about 5% of the functional anterior pituitary cells and are situated predominantly in the anteromedial areas of the gland. The hypothalamus senses low circulating levels of thyroid hormone (T3 and T4) and responds by releasing thyrotropin-releasing hormone (TRH) from the neuroendocrine cells of the paraventricular nucleus. The TRH stimulates the pituitary to produce thyroid-stimulating hormone (TSH). The TSH, in turn, stimulates the thyroid to produce thyroid hormone thyroxine (T4) and to a lesser degree, triiodothyronine (T3). The major portion of T3, however, is produced in peripheral organs, e.g. liver, adipose tissue, glia and skeletal muscle by deiodination from circulating T4. Deiodination is controlled by numerous hormones and nerve signals including TSH, vasopressin and catecholamines. Both peripheral thyroid hormones (iodothyronines) inhibit thyrotropin secretion from the pituitary (negative feedback). Consecutively, equilibrium concentrations for all hormones are attained.
Thyroid hormone exerts negative feedback control over the hypothalamus as well as anterior pituitary, thus controlling the release of both TRH from hypothalamus and TSH from anterior pituitary gland.
Anda mungkin juga menyukai
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Negative: What Does It Mean To Have A Test Result?Dokumen2 halamanNegative: What Does It Mean To Have A Test Result?Todd EddyBelum ada peringkat
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- Couple ReportDokumen10 halamanCouple ReportTrey TaylorBelum ada peringkat
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Heartfulness Magazine - January 2019 (Volume 4, Issue 1)Dokumen84 halamanHeartfulness Magazine - January 2019 (Volume 4, Issue 1)HeartfulnessBelum ada peringkat
- DRUG STUDY-1st BatchDokumen27 halamanDRUG STUDY-1st BatchCanny CańasBelum ada peringkat
- Resilience in Nurses - An Integrative ReviewDokumen15 halamanResilience in Nurses - An Integrative ReviewBob SmithBelum ada peringkat
- Etiology of Eating DisorderDokumen5 halamanEtiology of Eating DisorderCecillia Primawaty100% (1)
- TCA 88 Operating InstructionsDokumen276 halamanTCA 88 Operating InstructionsRonald Sol Salen Jordas80% (5)
- Test de Hormona ProlactinaDokumen31 halamanTest de Hormona ProlactinakemitaBelum ada peringkat
- Cierre Percutaneo de Pca en PrematurosDokumen12 halamanCierre Percutaneo de Pca en PrematurosNancy Romero QuirosBelum ada peringkat
- Risk Indicators and Interceptive Treatment Alternatives For Palatally Displaced Canines2010 - 16 - 3 - 186 - 192Dokumen7 halamanRisk Indicators and Interceptive Treatment Alternatives For Palatally Displaced Canines2010 - 16 - 3 - 186 - 192griffone1Belum ada peringkat
- Clinical Potings List 2021-22Dokumen1 halamanClinical Potings List 2021-22Dani ursBelum ada peringkat
- TTTTDokumen26 halamanTTTTMoataz TrabehBelum ada peringkat
- Public Warned vs. Chickenpox (Article From ABSCBN News) : ReflectionDokumen1 halamanPublic Warned vs. Chickenpox (Article From ABSCBN News) : Reflectionkuu faalBelum ada peringkat
- CH 04 The Skeletal System (New)Dokumen85 halamanCH 04 The Skeletal System (New)alyssa bananBelum ada peringkat
- Mr. Abhishek Bajpai Bajpai: Wellwise Advanced ProfileDokumen40 halamanMr. Abhishek Bajpai Bajpai: Wellwise Advanced ProfileAbhishek BajpaiBelum ada peringkat
- Sustaina-Loo Sanitation Solution EWB ChallengeDokumen83 halamanSustaina-Loo Sanitation Solution EWB ChallengeRichard WolfeBelum ada peringkat
- 5 Elements Qi GongDokumen1 halaman5 Elements Qi GongBill HonakerBelum ada peringkat
- 6 01 11Dokumen36 halaman6 01 11grapevineBelum ada peringkat
- 3-2. Assessing Production DocumentsDokumen48 halaman3-2. Assessing Production DocumentsSandeep sharmaBelum ada peringkat
- The Tongue - Facts, Function & Diseases - Live SciencerDokumen12 halamanThe Tongue - Facts, Function & Diseases - Live SciencerImtiax LaghariBelum ada peringkat
- Transesophageal Echocardiography: M. Elizabeth Brickner, MDDokumen9 halamanTransesophageal Echocardiography: M. Elizabeth Brickner, MDHilario. Hayascent.Reign.M.Belum ada peringkat
- Nursing 2 4yDokumen10 halamanNursing 2 4yGCON KURNOOLBelum ada peringkat
- Pathophys BURNDokumen2 halamanPathophys BURNpaupaulala83% (6)
- En Defensa de Los NiñosDokumen10 halamanEn Defensa de Los NiñosIliana GarcíaBelum ada peringkat
- Exercise For Specific Postural Variations in Pistol Shooting Part 2Dokumen3 halamanExercise For Specific Postural Variations in Pistol Shooting Part 2kholamonBelum ada peringkat
- Shell Gadus S3 Wirerope T: Performance, Features & Benefits Main ApplicationsDokumen2 halamanShell Gadus S3 Wirerope T: Performance, Features & Benefits Main ApplicationssfkcrcnBelum ada peringkat
- Document 2Dokumen9 halamanDocument 2zoha fatimaBelum ada peringkat
- Preservatives Used in Eye Drops: Paytaxt Private Institute Pharmacy Department 1 StageDokumen7 halamanPreservatives Used in Eye Drops: Paytaxt Private Institute Pharmacy Department 1 StageShakar Ezaddin AbdullahBelum ada peringkat
- Ashgate - Landscape Professional Practice PDFDokumen281 halamanAshgate - Landscape Professional Practice PDFyondaimethunderBelum ada peringkat
- PDF TextDokumen3 halamanPDF TextYogita PalBelum ada peringkat