IR Spectroscopy Basics
Diunggah oleh
coolhemakumarDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
IR Spectroscopy Basics
Diunggah oleh
coolhemakumarHak Cipta:
Format Tersedia
Infrared Spectroscopy Basics
IR Spectroscopy
I.
Introduction A. Spectroscopy is the study of the interaction of matter with the electromagnetic spectrum
1. 2. 3. Electromagnetic radiation displays the properties of both particles and waves The particle component is called a photon The energy (E) component of a photon is proportional to the frequency . Where h is Plancks constant and n is the frequency in Hertz (cycles per second) E = hn 4. The term photon is implied to mean a small, massless particle that contains a small wave-packet of EM radiation/light we will use this terminology in the course
IR Spectroscopy
I.
Introduction 5. Because the speed of light, c, is constant, the frequency, n, (number of cycles of the wave per second) can complete in the same time, must be inversely proportional to how long the oscillation is, or wavelength:
n=
c ___ l
E = hn =
c = 3 x 1010 cm/s
hc ___ l
6. 7.
Amplitude, A, describes the wave height, or strength of the oscillation Because the atomic particles in matter also exhibit wave and particle properties (though opposite in how much) EM radiation can interact with matter in two ways: Collision particle-to-particle energy is lost as heat and movement Coupling the wave property of the radiation matches the wave property of the particle and couple to the next higher quantum mechanical energy level
IR Spectroscopy
I.
Introduction 8. The entire electromagnetic spectrum is used by chemists: Frequency, n in Hz
~1019 ~1017 ~1015 ~1013 ~1010 ~105
Wavelength, l
~.0001 nm ~0.01 nm 10 nm 1000 nm 0.01 cm 100 m
Energy (kcal/mol)
> 300 300-30 300-30 ~10-4 ~10-6
g-rays
nuclear excitation (PET)
X-rays
core electron excitation (X-ray cryst.)
UV
electronic excitation (p to p*)
IR
molecular vibration
Microwave
molecular rotation
Radio
Nuclear Magnetic Resonance NMR (MRI)
Visible
IR Spectroscopy
I.
Introduction C. The IR Spectroscopic Process 1. The quantum mechanical energy levels observed in IR spectroscopy are those of molecular vibration 2.
3.
We perceive this vibration as heat
When we say a covalent bond between two atoms is of a certain length, we are citing an average because the bond behaves as if it were a vibrating spring connecting the two atoms For a simple diatomic molecule, this model is easy to visualize:
4.
IR Spectroscopy
I.
Introduction C. The IR Spectroscopic Process 5. There are two types of bond vibration: Stretch Vibration or oscillation along the line of the bond
H C H
symmetric
H C H
asymmetric
Bend Vibration or oscillation not along the line of the bond
H C H C H
rock
in plane
H C H
twist
H C
H
scissor
H
wag out of plane
Infrared Spectroscopy
C.The IR Spectroscopic Process 6.As a covalent bond oscillates due to the oscillation of the dipole of the molecule a varying electromagnetic field is produced
7.The greater the dipole moment change through the vibration, the more intense the EM field that is generated
Infrared Spectroscopy
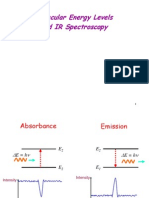
C. The IR Spectroscopic Process 8.When a wave of infrared light encounters this oscillating EM field generated by the oscillating dipole of the same frequency, the two waves couple, and IR light is absorbed
9.The coupled wave now vibrates with twice the amplitude
IR beam from spectrometer
coupled wave
EM oscillating wave from bond vibration
Infrared Spectroscopy
D.
The IR Spectrum 1. Each stretching and bending vibration occurs with a characteristic frequency as the atoms and charges involved are different for different bonds
The y-axis on an IR spectrum is in units of % transmittance In regions where the EM field of an osc. bond interacts with IR light of the same n transmittance is low (light is absorbed) In regions where no osc. bond is interacting with IR light, transmittance nears 100%
IR Spectroscopy
D.
The IR Spectrum 2. The x-axis of the IR spectrum is in units of wavenumbers, n, which is the number of waves per centimeter in units of cm-1 (Remember E = hn or E = hc/l)
IR Spectroscopy
D.
The IR Spectrum 3. This unit is used rather than wavelength (microns) because wavenumbers are directly proportional to the energy of transition being observed
chemists like this, physicists hate it
is quicker to understand than
High frequencies and high wavenumbers equate higher energy
Short wavelengths equate higher energy
4. 5. 6. This unit is used rather than frequency as the numbers are more real than the exponential units of frequency IR spectra are observed for what is called the mid-infrared: 400-4000 cm-1 The peaks are Gaussian distributions of the average energy of a transition
IR Spectroscopy
D.
The IR Spectrum 7. In general: Lighter atoms will allow the oscillation to be faster higher energy This is especially true of bonds to hydrogen C-H, N-H and O-H Stronger bonds will have higher energy oscillations Triple bonds > double bonds > single bonds in energy
Energy/n of oscillation
Infrared Spectroscopy
E.
The IR Spectrum The detection of different bonds 7. As opposed to chromatography or other spectroscopic methods, the area of a IR band (or peak) is not directly proportional to concentration of the functional group producing the peak
8. The intensity of an IR band is affected by two primary factors: Whether the vibration is one of stretching or bending Electronegativity difference of the atoms involved in the bond For both effects, the greater the change in dipole moment in a given vibration or bend, the larger the peak. The greater the difference in electronegativity between the atoms involved in bonding, the larger the dipole moment
Typically, stretching will change dipole moment more than bending
Infrared Spectroscopy
E.
The IR Spectrum The detection of different bonds 9. It is important to make note of peak intensities to show the effect of these factors:
Strong (s) peak is tall, transmittance is low (0-35 %) Medium (m) peak is mid-height (75-35%) Weak (w) peak is short, transmittance is high (90-75%) * Broad (br) if the Gaussian distribution is abnormally broad (*this is more for describing a bond that spans many energies) Exact transmittance values are rarely recorded
Infrared Spectroscopy
II.
Infrared Group Analysis A. General 1. The primary use of the IR spectrometer is to detect functional groups
2. Because the IR looks at the interaction of the EM spectrum with actual bonds, it provides a unique qualitative probe into the functionality of a molecule, as functional groups are merely different configurations of different types of bonds Since most types of bonds in covalent molecules have roughly the same energy, i.e., C=C and C=O bonds, C-H and N-H bonds they show up in similar regions of the IR spectrum Remember all organic functional groups are made of multiple bonds and therefore show up as multiple IR bands (peaks)
3.
4.
Infrared Spectroscopy
II.
Infrared Group Analysis A. General 5. The four primary regions of the IR spectrum
Bonds to H O-H single bond N-H single bond C-H single bond Triple bonds CC CN Double bonds C=O C=N C=C Single Bonds C-C C-N C-O Fingerprint Region
4000 cm-1
2700 cm-1
2000 cm-1
1600 cm-1
400 cm-1
Anda mungkin juga menyukai
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Instrumental Analysis Lecture Notes IIDokumen56 halamanInstrumental Analysis Lecture Notes IIcoolhemakumar100% (3)
- Instrument Control Uning LABVIEWDokumen69 halamanInstrument Control Uning LABVIEWcoolhemakumarBelum ada peringkat
- Instrumental Analysis Lecture Notes IIIDokumen62 halamanInstrumental Analysis Lecture Notes IIIcoolhemakumar100% (1)
- V / F ConverterDokumen8 halamanV / F ConvertercoolhemakumarBelum ada peringkat
- Interaction of EM Radiation and MatterDokumen3 halamanInteraction of EM Radiation and MattercoolhemakumarBelum ada peringkat
- LabVIEW IntroductionDokumen89 halamanLabVIEW Introductioncoolhemakumar100% (1)
- Basics of V/F and F/V ConvertersDokumen11 halamanBasics of V/F and F/V ConverterscoolhemakumarBelum ada peringkat
- Electromagnetic RadiationDokumen2 halamanElectromagnetic RadiationcoolhemakumarBelum ada peringkat
- Unit 7 Flame PhotometryDokumen32 halamanUnit 7 Flame PhotometryRia Agnez100% (8)
- Interfacing To Microprocessor ControllerDokumen14 halamanInterfacing To Microprocessor ControllercoolhemakumarBelum ada peringkat
- Mass SpectrometryDokumen19 halamanMass SpectrometrycoolhemakumarBelum ada peringkat
- Virtual Instrumentation ArchitectureDokumen25 halamanVirtual Instrumentation Architecturecoolhemakumar100% (8)
- Molecular Energy Levels and IR SpectrosDokumen27 halamanMolecular Energy Levels and IR SpectroscoolhemakumarBelum ada peringkat
- Instrumental Methods of Analysis Lesson NotesDokumen80 halamanInstrumental Methods of Analysis Lesson NotescoolhemakumarBelum ada peringkat
- Digital and Pulse-Train Conditioning: Digital I/O InterfacingDokumen6 halamanDigital and Pulse-Train Conditioning: Digital I/O InterfacingcoolhemakumarBelum ada peringkat
- Energy Levels and Transitions in AtomsDokumen7 halamanEnergy Levels and Transitions in AtomscoolhemakumarBelum ada peringkat
- Virtual Instrumentation SystemsDokumen7 halamanVirtual Instrumentation SystemscoolhemakumarBelum ada peringkat
- Displacement and PositionDokumen6 halamanDisplacement and PositioncoolhemakumarBelum ada peringkat
- NMR Spectroscopy - Short NoteDokumen6 halamanNMR Spectroscopy - Short Notecoolhemakumar100% (4)
- NMR SpectrometerDokumen13 halamanNMR SpectrometercoolhemakumarBelum ada peringkat
- Automation in DVMsDokumen7 halamanAutomation in DVMscoolhemakumarBelum ada peringkat
- What Is Modular InstrumentationDokumen7 halamanWhat Is Modular InstrumentationcoolhemakumarBelum ada peringkat
- CASE ToolsDokumen5 halamanCASE ToolscoolhemakumarBelum ada peringkat
- Mass SpecDokumen38 halamanMass SpecRashi VermaBelum ada peringkat
- NMRDokumen142 halamanNMRMaulana Senjaya SusiloBelum ada peringkat
- Electron Spin ResonanceDokumen29 halamanElectron Spin Resonancecoolhemakumar100% (2)
- Mass Spectrometry - Short NoteDokumen2 halamanMass Spectrometry - Short NotecoolhemakumarBelum ada peringkat
- Hart TutorialDokumen6 halamanHart TutorialabhiklBelum ada peringkat
- AN713 - Controller Area Network (CAN) BasicsDokumen9 halamanAN713 - Controller Area Network (CAN) BasicsRam Krishan SharmaBelum ada peringkat
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- As 60269.2.1-2005 Low-Voltage Fuses - Supplementary Requirements For Fuses For Use by Authorized Persons (FusDokumen12 halamanAs 60269.2.1-2005 Low-Voltage Fuses - Supplementary Requirements For Fuses For Use by Authorized Persons (FusSAI Global - APACBelum ada peringkat
- Plaxis Fahmi Arief Penarosa F11222034Dokumen13 halamanPlaxis Fahmi Arief Penarosa F11222034Andi Azis RusdiBelum ada peringkat
- Fundamentals of Hydrogen Embrittlement 2016Dokumen241 halamanFundamentals of Hydrogen Embrittlement 2016Jorge Echeverri100% (1)
- APCalAB - PT01 - Section1 - Part BDokumen10 halamanAPCalAB - PT01 - Section1 - Part BSurgaltiin Alba MBparkBelum ada peringkat
- P3800 Series: High Pressure Hydraulic Deadweight Testers Models P3830, P3840 and P3860Dokumen2 halamanP3800 Series: High Pressure Hydraulic Deadweight Testers Models P3830, P3840 and P3860vidhyananthanBelum ada peringkat
- Oil Filter Inspection - What To Look ForDokumen7 halamanOil Filter Inspection - What To Look ForchrisBelum ada peringkat
- 5660279Dokumen31 halaman5660279DanishwarBelum ada peringkat
- Structural Analysis Truss ForcesDokumen36 halamanStructural Analysis Truss ForcesAngela MurielBelum ada peringkat
- Deltabeam EngDokumen20 halamanDeltabeam EngAmir OmeraševićBelum ada peringkat
- Human Eye and The Colourful WorldDokumen2 halamanHuman Eye and The Colourful WorldVenu GopalBelum ada peringkat
- How Do Study PhysicsDokumen12 halamanHow Do Study Physicslim chuan yangBelum ada peringkat
- Ian Stewart's Nature's Number Synthesis PaperDokumen3 halamanIan Stewart's Nature's Number Synthesis PaperGeorgette Matt74% (61)
- IEEE STD ANSI-IEEE STD 116-1975Dokumen12 halamanIEEE STD ANSI-IEEE STD 116-1975abdou samiBelum ada peringkat
- 731 2545 1 PBDokumen6 halaman731 2545 1 PBAnjas ABBelum ada peringkat
- Eddy Current Testing Technology - 2nd Edition - SampleDokumen22 halamanEddy Current Testing Technology - 2nd Edition - Samplecopica ultrasonidoBelum ada peringkat
- Composite FloorsDokumen22 halamanComposite Floorsامين الزريقيBelum ada peringkat
- Dissecting Hydrometer CalculationsDokumen5 halamanDissecting Hydrometer CalculationsJose Miguel Romero SevillaBelum ada peringkat
- Failure Analysis of Mixed Mode Crack Growth in Heavy Duty Truck Frame RailDokumen8 halamanFailure Analysis of Mixed Mode Crack Growth in Heavy Duty Truck Frame RailnaderbahramiBelum ada peringkat
- Ratio and Proportion Worksheets 7th Grade Worksheet 3Dokumen9 halamanRatio and Proportion Worksheets 7th Grade Worksheet 3Rashida ArshadBelum ada peringkat
- 1796 Books Doubtnut Question BankDokumen16 halaman1796 Books Doubtnut Question BankMohit BhatiBelum ada peringkat
- CFD FDP Online Five-Day ScheduleDokumen1 halamanCFD FDP Online Five-Day ScheduleSujit MishraBelum ada peringkat
- CHAPTER 2: MOTION & ENERGY VECTORSDokumen33 halamanCHAPTER 2: MOTION & ENERGY VECTORSMahmoud SamahinBelum ada peringkat
- Oiml R 33: Nternational EcommendationDokumen9 halamanOiml R 33: Nternational EcommendationAhmad Atsari SujudBelum ada peringkat
- Hardness Test Report 2021Dokumen6 halamanHardness Test Report 2021Md. Nahin Al ZakiBelum ada peringkat
- Data Sheet FLC 100Dokumen2 halamanData Sheet FLC 100Ömer Vehbe100% (1)
- Problems For PH1016: Chapter 31: 37, 41, 43, 45 (Op.), 57, 59, 61 (Op.) Chapter 32. 35 (Op.), 37, 39, 45, 47, 49, 51Dokumen9 halamanProblems For PH1016: Chapter 31: 37, 41, 43, 45 (Op.), 57, 59, 61 (Op.) Chapter 32. 35 (Op.), 37, 39, 45, 47, 49, 51Vũ Đức TuânBelum ada peringkat
- ASTM A36-A36M-08 Standard Specification For Carbon Structural SteelDokumen3 halamanASTM A36-A36M-08 Standard Specification For Carbon Structural SteelAarón Escorza MistránBelum ada peringkat
- Analisa Unjuk Kerja Mesin Stempel Flash Made in Tiongkok Merek Flaz Terhadap Mesin Stempel Flash Made in Indonesia Merek MDDokumen6 halamanAnalisa Unjuk Kerja Mesin Stempel Flash Made in Tiongkok Merek Flaz Terhadap Mesin Stempel Flash Made in Indonesia Merek MDbinangkit androidBelum ada peringkat
- Avogardo Goes To Court Case StudyDokumen6 halamanAvogardo Goes To Court Case StudyeBelum ada peringkat
- Cambridge O Level: Additional Mathematics 4037/13 October/November 2022Dokumen10 halamanCambridge O Level: Additional Mathematics 4037/13 October/November 2022MolfBelum ada peringkat