Nuclear Lecture
Diunggah oleh
Ronalson SiraitDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Nuclear Lecture
Diunggah oleh
Ronalson SiraitHak Cipta:
Format Tersedia
1
Nuclear Chemistry
Chemistry I Chapter 25
Chemistry I Honors
Chapter 19
ICP Chapter 18
SAVE PAPER AND INK!!! When you
print out the notes on PowerPoint,
print "Handouts" instead of
"Slides" in the print setup. Also,
turn off the backgrounds
(Tools>Options>Print>UNcheck
"Background Printing")!
2
Radioactivity
One of the pieces of evidence for the
fact that atoms are made of smaller
particles came from the work of
________ (1876-1934).
She discovered ________, the
spontaneous disintegration of some
elements into smaller pieces.
3
Nuclear Reactions vs.
Normal Chemical Changes
Nuclear reactions involve the nucleus
The nucleus opens, and protons and
neutrons are rearranged
The opening of the nucleus releases a
tremendous amount of energy that holds
the nucleus together called binding
energy
Normal Chemical Reactions involve
electrons, not protons and neutrons
4
Mass Defect
Some of the mass can be converted into
energy
Shown by a very famous equation!
E=mc
2
Energy
Mass
Speed of light
5
Types of Radiation
e
0
1
He
4
2
Alpha () a positively
charged helium isotope - we
usually ignore the charge because it involves
electrons, not protons and neutrons
Beta () an electron
Gamma () pure energy;
called a ray rather than a
particle
0
0
6
Other Nuclear Particles
e
0
1
n
1
0
Neutron
Positron a positive
electron
Proton usually referred to
as hydrogen-1
Any other elemental isotope
H
1
1
7
Penetrating Ability
8
Balancing Nuclear Reactions
In the reactants (starting materials
on the left side of an equation) and
products (final products on the right
side of an equation)
Atomic numbers must balance
and
Mass numbers must balance
Use a particle or isotope to fill in the
missing protons and neutrons
9
Nuclear Reactions
Alpha emission
Note that mass number (A) goes down by 4
and atomic number (Z) goes down by 2.
Nucleons (nuclear particles protons and
neutrons) are rearranged but conserved
10
Nuclear Reactions
Beta emission
Note that mass number (A) is unchanged
and atomic number (Z) goes up by 1.
11
Other Types of Nuclear Reactions
Positron (
0
+1
b): a positive electron
Electron capture: the capture of an electron
207 207
12
Learning Check
What radioactive isotope is produced in the
following bombardment of boron?
10
B +
4
He ? +
1
n
5 2 0
13
Write Nuclear Equations!
Write the nuclear equation for the beta
emitter Co-60.
14
Artificial Nuclear Reactions
New elements or new isotopes of known elements
are produced by bombarding an atom with a
subatomic particle such as a proton or neutron
-- or even a much heavier particle such as
4
He
and
11
B.
Reactions using neutrons are called
reactions because a ray is usually
emitted.
Radioisotopes used in medicine are often made by
reactions.
15
Artificial Nuclear Reactions
Example of a reaction is production
of radioactive
31
P for use in studies of P
uptake in the body.
31
15
P +
1
0
n --->
32
15
P +
16
Transuranium Elements
Elements beyond 92 (transuranium) made
starting with an reaction
238
92
U +
1
0
n --->
239
92
U +
239
92
U --->
239
93
Np +
0
-1
b
239
93
Np --->
239
94
Pu +
0
-1
b
17
Nuclear Fission
18
Nuclear Fission
Fission is the splitting of atoms
These are usually very large, so that they are not as stable
Fission chain has three general steps:
1. Initiation. Reaction of a single atom starts the
chain (e.g.,
235
U + neutron)
2. Propagation.
236
U fission releases neutrons that
initiate other fissions
3. ___________ .
19
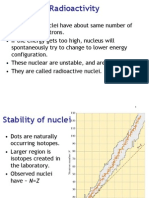
Stability
of Nuclei
Out of > 300 stable isotopes:
Even
Odd
Odd
Even
Z
N
157 52
50 5
31
15
P
19
9
F
2
1
H,
6
3
Li,
10
5
B,
14
7
N,
180
73
Ta
20
Band of Stability
and Radioactive
Decay
21
Representation of a fission process.
22
Nuclear Fission & POWER
Currently about 103
nuclear power plants in
the U.S. and about 435
worldwide.
17% of the worlds
energy comes from
nuclear.
23
Figure 19.6: Diagram of a nuclear power plant.
24
Nuclear Fusion
Fusion
small nuclei combine
2
H +
3
H
4
He +
1
n +
1 1 2 0
Occurs in the sun and other stars
Energy
25
Nuclear Fusion
Fusion
Excessive heat can not be contained
Attempts at cold fusion have
FAILED.
Hot fusion is difficult to contain
26
Half-Life
HALF-LIFE is the time that it takes for
1/2 a sample to decompose.
The rate of a nuclear transformation
depends only on the reactant
concentration.
27
Half-Life
Decay of 20.0 mg of
15
O. What remains after 3 half-lives?
After 5 half-lives?
28
Kinetics of Radioactive Decay
For each duration (half-life), one half of the
substance decomposes.
For example: Ra-234 has a half-life of 3.6 days
If you start with 50 grams of Ra-234
After 3.6 days > 25 grams
After 7.2 days > 12.5 grams
After 10.8 days > 6.25 grams
29
Learning Check!
The half life of I-123 is 13 hr. How much of
a 64 mg sample of I-123 is left after 39
hours?
30
Effects of Radiation
31
Geiger Counter
Used to detect radioactive substances
32
33
Radiocarbon Dating
Radioactive C-14 is formed in the upper atmosphere
by nuclear reactions initiated by neutrons in
cosmic radiation
14
N +
1
o
n --->
14
C +
1
H
The C-14 is oxidized to CO
2
, which circulates
through the biosphere.
When a plant dies, the C-14 is not replenished.
But the C-14 continues to decay with t
1/2
= 5730
years.
Activity of a sample can be used to date the sample.
34
Nuclear Medicine: Imaging
Thyroid imaging using Tc-99m
35
Food Irradiation
Food can be irradiated with rays from
60
Co or
137
Cs.
Irradiated milk has a shelf life of 3 mo.
without refrigeration.
USDA has approved irradiation of meats
and eggs.
Anda mungkin juga menyukai
- Practice Makes Perfect in Chemistry: Nuclear EnergyDari EverandPractice Makes Perfect in Chemistry: Nuclear EnergyPenilaian: 5 dari 5 bintang5/5 (1)
- 6 CH 19 Nuclear ChemistryDokumen35 halaman6 CH 19 Nuclear ChemistryFatin IziantiBelum ada peringkat
- Practice Makes Perfect in Chemistry: Nuclear Chemistry with AnswersDari EverandPractice Makes Perfect in Chemistry: Nuclear Chemistry with AnswersPenilaian: 5 dari 5 bintang5/5 (1)
- Student CH 19 NuclearDokumen34 halamanStudent CH 19 NuclearMaheshBelum ada peringkat
- Kimia Inti: Nuclear ChemistryDokumen39 halamanKimia Inti: Nuclear Chemistrydimasaditya28Belum ada peringkat
- Chapter 5 NuclearDokumen21 halamanChapter 5 NuclearUrooj GulBelum ada peringkat
- Chem 114-Nuclear ChemistryDokumen36 halamanChem 114-Nuclear ChemistryKaizBelum ada peringkat
- Lecture 3 Nuclear ChemistryDokumen52 halamanLecture 3 Nuclear ChemistryGuilbert FajardoBelum ada peringkat
- 4 Nuclear Chemistry (Type of Radioactive Decay)Dokumen15 halaman4 Nuclear Chemistry (Type of Radioactive Decay)Fatin IziantiBelum ada peringkat
- Nuclear Reactions: William L Masterton Cecile N. Hurley Edward J. NethDokumen68 halamanNuclear Reactions: William L Masterton Cecile N. Hurley Edward J. NethJulieAnne DelaCruzBelum ada peringkat
- Notes On Nuclear ChemistryDokumen1 halamanNotes On Nuclear Chemistryanon_2475374Belum ada peringkat
- Chapter 23: Nuclear Chemistry: - Nuclear Changes - Nuclear Equations - U TH+ HeDokumen72 halamanChapter 23: Nuclear Chemistry: - Nuclear Changes - Nuclear Equations - U TH+ HeNancy SantosBelum ada peringkat
- Nuclear RadiationDokumen26 halamanNuclear RadiationosamaalazbBelum ada peringkat
- Nuclear DecayDokumen34 halamanNuclear DecayAyya ChannBelum ada peringkat
- Nuclear PhysicsDokumen24 halamanNuclear PhysicsCalvin LabialBelum ada peringkat
- Group3 Rad106Dokumen50 halamanGroup3 Rad106chacha 7074684Belum ada peringkat
- MODULE 3 Nuclear ChemistryDokumen28 halamanMODULE 3 Nuclear ChemistryILIVEFOR MONSTA7Belum ada peringkat
- Lesson 2. Nuclear Chemistry EnergyDokumen12 halamanLesson 2. Nuclear Chemistry EnergyKate ComonicalBelum ada peringkat
- Nuclear ChemDokumen31 halamanNuclear ChemAashish SapkotaBelum ada peringkat
- 02 Lesson 2. Nuclear Chemistry & EnergyDokumen12 halaman02 Lesson 2. Nuclear Chemistry & EnergyBianca VacunawaBelum ada peringkat
- Miss Rabia Najam: Nuclear PHYSICS Presentation Submitted ToDokumen13 halamanMiss Rabia Najam: Nuclear PHYSICS Presentation Submitted ToWajeeha IftikharBelum ada peringkat
- LecPPT3 - Neutron Interactions, Cross-Section and ExamplesDokumen32 halamanLecPPT3 - Neutron Interactions, Cross-Section and ExamplesVishal MeenaBelum ada peringkat
- Nuclear and Radiation 100LDokumen12 halamanNuclear and Radiation 100Lekanadefestus007Belum ada peringkat
- Chapter 2 Nuclear ChemistryDokumen26 halamanChapter 2 Nuclear ChemistryJakeNK94Belum ada peringkat
- Physics 10 ExamDokumen27 halamanPhysics 10 ExamPreston LimeBelum ada peringkat
- Nuclear EquationsDokumen21 halamanNuclear EquationsAbigail AmorBelum ada peringkat
- Nuclear ChemistryDokumen42 halamanNuclear ChemistryJann Romene Decena100% (1)
- 2-Basic Concepts of Nuclear Physics and Overview of ReactorDokumen67 halaman2-Basic Concepts of Nuclear Physics and Overview of ReactorswamikashyapBelum ada peringkat
- Lecture 5Dokumen18 halamanLecture 5Marielle BrionesBelum ada peringkat
- Target: Did You Know? Positron-Emission Tomography (PET) Scans, in Figure 1, Shows TheDokumen10 halamanTarget: Did You Know? Positron-Emission Tomography (PET) Scans, in Figure 1, Shows TheGrace AmarBelum ada peringkat
- UNIT 5 NcesDokumen100 halamanUNIT 5 NcesSHAIKJAFARBelum ada peringkat
- Nuclear ChemistryDokumen9 halamanNuclear Chemistrysalinips3Belum ada peringkat
- CFE - 11 Nuclear ChemistryDokumen37 halamanCFE - 11 Nuclear ChemistryChristian C. Suase100% (1)
- KS4 Radioactive DecayDokumen31 halamanKS4 Radioactive DecayJaleel James100% (1)
- Chemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 2Dokumen13 halamanChemistry For Engineers 1 Energy Topic 04 Nuclear Chemistry 2Kristine AlcantaraBelum ada peringkat
- Nuclear ChemistryDokumen33 halamanNuclear ChemistryPRIYA ManobanBelum ada peringkat
- Nuclear ChemistryDokumen2 halamanNuclear ChemistryAyan BarbonBelum ada peringkat
- Nuclear Transmutation OL2Dokumen33 halamanNuclear Transmutation OL2Shifa ChaudhariBelum ada peringkat
- Chapter 19 Notes - Nuclear ChemistryDokumen13 halamanChapter 19 Notes - Nuclear ChemistryDavid StainesBelum ada peringkat
- 13 ChangesInTheNucleus 2bDokumen14 halaman13 ChangesInTheNucleus 2bmainethemaineBelum ada peringkat
- Chpater 14 Nuclear ChemistryDokumen53 halamanChpater 14 Nuclear ChemistryAndrearose Ivy FietasBelum ada peringkat
- 2 - Nuc ChemDokumen44 halaman2 - Nuc ChemRENIER JANE GAIDBelum ada peringkat
- Nuclear ChemDokumen46 halamanNuclear ChemchimBelum ada peringkat
- Nuclear Chemistry and Applications of RadioactivityDokumen15 halamanNuclear Chemistry and Applications of RadioactivityAnusha KhadkaBelum ada peringkat
- RadioactivityDokumen31 halamanRadioactivityRamliRemBelum ada peringkat
- UntitledDokumen36 halamanUntitledahsan shahBelum ada peringkat
- 1 - Nuclear Chemistry PDFDokumen51 halaman1 - Nuclear Chemistry PDFSangga Buana Komara100% (1)
- NucleiDokumen32 halamanNucleiGDGGFGFBelum ada peringkat
- Physics: The Study of MatterDokumen15 halamanPhysics: The Study of MatterTaha RashidBelum ada peringkat
- Nuclear Chemistry: Symbol or Symbol - Mass #Dokumen18 halamanNuclear Chemistry: Symbol or Symbol - Mass #simpanan hitamBelum ada peringkat
- Module 3 Nuclear ReactionDokumen15 halamanModule 3 Nuclear ReactionShannBelum ada peringkat
- CHEM 357 - 24 - Lecture 5-6Dokumen33 halamanCHEM 357 - 24 - Lecture 5-6Abede Saviour DelaliBelum ada peringkat
- ENCHML130 1 Energy 5 Nuclear 2Dokumen47 halamanENCHML130 1 Energy 5 Nuclear 2G7 SJ-01 Cabataña, MichailaBelum ada peringkat
- 16 Nuclear1Dokumen25 halaman16 Nuclear1Godfrey KayomboBelum ada peringkat
- Radioactivity NotesDokumen16 halamanRadioactivity NotesNooruddin SheikBelum ada peringkat
- Nuclear ChemistryDokumen47 halamanNuclear ChemistryEmman Revilla100% (3)
- Revision Notes For NucleationDokumen7 halamanRevision Notes For Nucleationsumakodipaka938Belum ada peringkat
- Chm524 4 RadiochemistryDokumen67 halamanChm524 4 Radiochemistryasyraf azlan99Belum ada peringkat
- Lec 6 - Nuclear ChemistryDokumen54 halamanLec 6 - Nuclear ChemistryCarlo EstoleBelum ada peringkat
- CH 15Dokumen53 halamanCH 15Ronalson SiraitBelum ada peringkat
- How To Report and Use UncertaintiesDokumen22 halamanHow To Report and Use UncertaintiesRonalson SiraitBelum ada peringkat
- Measurement & Uncertainties: Dosen PengampuDokumen26 halamanMeasurement & Uncertainties: Dosen PengampuRonalson SiraitBelum ada peringkat
- chapter2MPF2012 PDFDokumen80 halamanchapter2MPF2012 PDFRonalson SiraitBelum ada peringkat
- General Structure of Wave Mechanics (Ch. 5) : Product Dot DX U U U U J I X U Ions EigenfunctDokumen10 halamanGeneral Structure of Wave Mechanics (Ch. 5) : Product Dot DX U U U U J I X U Ions EigenfunctRonalson SiraitBelum ada peringkat
- Emulsi Pada Produk SusuDokumen28 halamanEmulsi Pada Produk SusuRonalson SiraitBelum ada peringkat
- Christopher Chui: 6/5/2014 Nuclear Chemistry - C. Chui 1Dokumen11 halamanChristopher Chui: 6/5/2014 Nuclear Chemistry - C. Chui 1Ronalson SiraitBelum ada peringkat
- Deterministic ModelsDokumen1 halamanDeterministic ModelsRonalson SiraitBelum ada peringkat
- Bab 3 Pengantar Inferensi StatistikaDokumen46 halamanBab 3 Pengantar Inferensi StatistikaRonalson SiraitBelum ada peringkat
- ANTARES Cased Hole Instrument Catalog 2019sDokumen29 halamanANTARES Cased Hole Instrument Catalog 2019sJuan Angel Gonzalez CamposBelum ada peringkat
- Atoms and Molecules: Larry Brown Tom HolmeDokumen62 halamanAtoms and Molecules: Larry Brown Tom Holmemuhammad ali shakeelBelum ada peringkat
- Full Download Human Physiology 13th Edition Stuart Ira Fox Test BankDokumen35 halamanFull Download Human Physiology 13th Edition Stuart Ira Fox Test Bankgambolrapinous.ggqcdr100% (40)
- CHEM 1411 Chapter 5 Homework AnswersDokumen8 halamanCHEM 1411 Chapter 5 Homework AnswersGrothendieck Langlands ShtukasBelum ada peringkat
- Loeblein Chemistry Clicker Questions2013 PDFDokumen279 halamanLoeblein Chemistry Clicker Questions2013 PDFCarolay Gabriela Aponte RodriguezBelum ada peringkat
- Fundamentals: - Neutron Logs: Are Porosity Logs That Measure The Hydrogen Concentration in A FormationDokumen20 halamanFundamentals: - Neutron Logs: Are Porosity Logs That Measure The Hydrogen Concentration in A FormationMonsterBelum ada peringkat
- Chem 101 Ex1 Review QuestionsDokumen35 halamanChem 101 Ex1 Review QuestionsJohn Lloyd PaduaBelum ada peringkat
- Nuclear Fission and Fusion - AnswersDokumen8 halamanNuclear Fission and Fusion - AnswersJJ FajardoBelum ada peringkat
- Angelica Pazziuagan, Answer Sheet Module 3 Part 1Dokumen4 halamanAngelica Pazziuagan, Answer Sheet Module 3 Part 1angelica pazziuaganBelum ada peringkat
- Self-Learning Home Task (SLHT)Dokumen6 halamanSelf-Learning Home Task (SLHT)Jim Alesther LapinaBelum ada peringkat
- Unit 01 Basic Chemistry Notes (Answers)Dokumen0 halamanUnit 01 Basic Chemistry Notes (Answers)sunilk09Belum ada peringkat
- Physics MathematicsDokumen36 halamanPhysics Mathematicsmudabbir muhammad33% (3)
- Pocket PhysicsDokumen10 halamanPocket PhysicsFaraz KhanBelum ada peringkat
- KALVI KADAL 12th Physics Important Questions 2020-21 EM WWW - Kalvikadal.inDokumen14 halamanKALVI KADAL 12th Physics Important Questions 2020-21 EM WWW - Kalvikadal.inIQAC - M.U.CollegeBelum ada peringkat
- CW 2 Atoms Short AnswersDokumen5 halamanCW 2 Atoms Short Answersmohammad hasanBelum ada peringkat
- Worksheet Investigating Decay ChainsDokumen8 halamanWorksheet Investigating Decay Chainsashton smithBelum ada peringkat
- Practice Exam Questions: Unit 4Dokumen2 halamanPractice Exam Questions: Unit 4alamphyBelum ada peringkat
- 1.1 Atomic Structure Test Mark Scheme: Allow No. of Nucleons / Amount of P & NDokumen3 halaman1.1 Atomic Structure Test Mark Scheme: Allow No. of Nucleons / Amount of P & NalexisBelum ada peringkat
- Atomic Structure Short Notes 7 PageDokumen7 halamanAtomic Structure Short Notes 7 PageSubhajit GoraiBelum ada peringkat
- Mass-Energy EquivalenceDokumen25 halamanMass-Energy EquivalenceSohail KhanBelum ada peringkat
- Liquid Metal Fast Breeder Reactor: Mona Mary Varghese Reg. No: Gcaocap008Dokumen19 halamanLiquid Metal Fast Breeder Reactor: Mona Mary Varghese Reg. No: Gcaocap008MONA MARYBelum ada peringkat
- Chapter 2Dokumen1 halamanChapter 2viji. viswanadhBelum ada peringkat
- Subject: Medical Physics Class: DPT 2 SemisterDokumen19 halamanSubject: Medical Physics Class: DPT 2 SemisterZuhaib AhmedBelum ada peringkat
- PHY Nuclear Fusion and FissionDokumen36 halamanPHY Nuclear Fusion and FissionSarika KandasamyBelum ada peringkat
- Heritage International School, Tala Nagri, Aligarh PRE-BOARD - I (2021-22) Chemistry XIIDokumen5 halamanHeritage International School, Tala Nagri, Aligarh PRE-BOARD - I (2021-22) Chemistry XIIBhookha bookishBelum ada peringkat
- PS - Q1 - Week 1aDokumen9 halamanPS - Q1 - Week 1aShekaina Faith Cuizon LozadaBelum ada peringkat
- QuimicaDokumen260 halamanQuimicaLuis MataBelum ada peringkat
- Learning The Periodic Table of ElementsDokumen31 halamanLearning The Periodic Table of ElementshypezakramBelum ada peringkat
- Chapter 1Dokumen15 halamanChapter 1BACHA NEGERIBelum ada peringkat