Isolasi & Identifikasi Alkaloid
Diunggah oleh
Fraztya HebbyHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Isolasi & Identifikasi Alkaloid
Diunggah oleh
Fraztya HebbyHak Cipta:
Format Tersedia
Cara Pembuatan Simplisia
1. Pemanenan
2. Pengumpulan
3. Sortasi basah
4. Pencucian
5. Perajangan
6. Pengeringan
7. Sortasi kering
8. Pengawetan
9. Pengemasan
Pada saat
pemanenan,
pengumpulan, dan
sortasi basah
Perhatikan garis-
garis besar
pemanenan.
Pencucian dengan air
mengalir untuk
mengilangkan debu
serta pengotor yang
melekat
Sesudah dicuci, bahan
diangin-anginkan
untuk mengurangi air
yang menempel.
Pengeringan, dapat
dilakukan di
1. Almari pengering
2. Oven
3. Sinar matahari
Pengemasan untuk mempertahankan mutu
simplisia dalam penyimpanan.
Yang menjadi perhatian:
1. Pedoman panen
2. Pengirisan bahan sebelum dikeringkan,
untuk bahan yang berukuran besar.
3. Pengeringan,
* di dalam oven
* di dalam almari pengering
* di bawah sinar matahari
Isolasi & Identifikasi alkaloid
dari Akar Pasak Bumi
(Eurycoma longifolia Jack)
Nina Salamah (PFA/561)
Eurycoma longifolia Jack leaves and fruits
Eurycoma longifolia Jack 6-years old plant and roots
Eurycoma longifolia Jack
Akar Pasak Bumi (Eurycoma longifolia
Jack) merupakan family Simaroubaceae
dikenal sebagai obat kuat (Tongkat Ali).
Tanaman ini tumbuh di hutan Indonesia
(Kalimantan) juga di Malaysia
Di Asia Tenggara digunakan untuk
antimalaria, antipyretic, antiulcer,
cytotoxic dan aphrodisiaka.
Eurycoma longifolia Jack
Menurut kardono ada 5 komponen aktif akar pasak bumi
yang berpotensi sitotoksik yaitu: 4 merupakan gol
alkaloid canthin-6-one (9-methoxycanthin-6-one, 9-
methoxycanthin-6-one-Noxide, 9-hydroxycanthin-6-
one, 9-hydroxycanthin-6-one-N-oxide) dan 1 golongan
quassinoid (eurycomanon).
Ke 5 senyawa diuji sitotoksiknya pada beberapa tipe sel
kanker [breast, colon, fibrosarcoma, lung, melanoma,
KB, and KB-V1 (a multi-drug resistant cell line derived
from KB)] and murine lymphocytic leukemia (P-388)
The canthin-6-ones 1-4 were found to be active with all
cell lines tested except for the KB-V 1 cell line.
Eurycomanone was inactive against murine lymphocytic
leukemia (P-388) but was significantly active against the
human cell lines tested.
Alkaloid canthin-6-one
9-methoxycanthin-6-one [1],
9-methoxycanthin-6-one-N-oxide [2],
9-hydroxycanthin-6-one [3], and
9-hydroxycanthin-6-one-N-oxide [4]
Riset
Isolasi senyawa (1) dan
(3) untuk dikembangkan
sebagai obat anti kanker
Dari strukturnya, kedua
senyawa ini mempunyai
sifat yang kurang polar.
Perlu dipisahkan dari
senyawa lain yang
berbeda kepolarannya
Cara isolasi
100 g serbuk akar kemudian perkolasi dengan
MeOH ekstrak MeOH CC silica gel
dengan fase gerak CHCL3:MeOH dalam berbagai
perbandingan 9 fraksi (F002-F010)
Fraksi 003 CC si gel ( CHCl3 :Me OH=
99:2) 9 methoksi canthin 6 one
Fraksi 006 CC Si Gel ( CHCl3 : MeOH =
96:4) 9 hidroksi canthin 6 one
evaporasi
Maserasi berulang dg etanol 50%
UV
FTIR
GC/MS
NMR
Simplisia Pasak Bumi
Maserat
Crude Ekstrak
n-Butanol
Dietil eter
Kolom Kromatografi
800 ml n-heksane:metanol
7:3 1:1 3:7
Purifikasi dengan HPLC semi preparatif Kolom C18,
2 ml/menit, Deteksi UV 240 nm
Acetonitril : air (3:7)
eurycomalacton 9-methoxycanthin-6-one
Fase gerak
Proses KVC
Diameter kolom = 5 cm
Berat silika = 30 70 g
(tinggi silika = 5 -6 cm)
Kapasitas sampel = 5 g
Volume eluen = 50 75 mL
Kunci
pemisahan/pemurnian yang
baik, pada pemilihan eluen
(fase gerak).
PREPARASI KOLOM
9-methoxycanthin-6-one
Identifiksi kualitatif:
KLT dengan CHCl3-
MeOH (98:2) as the
solvent system,
indicated further their
co-identity (Rf O. 50)
N
N
H
3
CO
O
senyawa 1
9-methoxy-6H-indolo[3,2,1-ij][1,5]naphthyridin-6-one
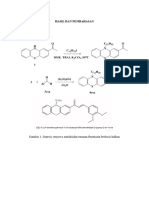
Tabel 1.
13
C-nmr Assignments for
Compounds 1
compound 1 was directly
comparable (mmp, uv, ir, 'H-
nmr, 13C nmr, ms, co-tlc) to
an isolate obtained earlier in
this laboratory from the wood
of S. multiflora, which was
assigned as 10-methoxy
canthin-6-one using primarily
low resolution 'H-nmr
spectroscopy (14). Therefore,
this S. multiflora isolate has
now been reassigned as 9-
methoxy canthin-6-one.
Carbon ppm
C-1
C-2
C-4
C-5
C-6
C-8
C-9
C-10
C-11
C-12
C-13
C-14
C-15
C-16
O-CH3
115.59
146.02
139.92
128.56
159.61
101.41
163.20
114.26
123.47
117.03
132.02
129.24
131.16
136.04
56.03
9-hydroxycanthin-6-one
Identifikasi kualitatif
Si gel tlc migration
data (Rf 0.40), using
CHCl,-MeOH (95:5).
N
N
HO
O
senyawa 3
9-hydroxy-6H-indolo[3,2,1-ij][1,5]naphthyridin-6-one
Tabel 2.
13
C dan
1
H-nmr
Assignments for Compounds 3
Carbon ppm
C-1
C-2
C-4
C-5
C-6
C-8
C-9
C-10
C-11
C-12
C-13
C-14
C-15
C-16
O-CH3
116.01
145.98
140.03
128.04
158.94
102.98
160.50
114.00
124.65
115.57
140.53
129.88
131.66
135.01
-
hidrogen ppm
H-1
H-2
H-4
H-5
H-8
H-10
H-11
O-CH3
8.14(d, 5.0)
8.76(d, 5.0)
8.10(d, 10)
6.96(d, 10)
8.00(d, 2.5)
7.00(dd, 9.2.5)
8.17 (d, 9)
-
13
C-NMR dan
1
H-NMR
'H- and 13C-nmr resonances of this compound were
quite comparable to those obtained for compound 1.
After measuring the 'H-'H COSY and 'H-13C HETCOR
nmr spectra of 3 and following comparison with
published unambiguous 'H- and 13C-nmr assignments
for canthin-6-one (17), the hydroxy group was
tentatively placed at C-9. In a selective INEPT
experiment (J
CH
= 8 Hz) performed with 3, irradiation of
H-2 ( 8.76) enhanced the resonances of C-14 (
129.88) and C-16 ( 135.03), while irradiation of H-11
( 8.17) enhanced the resonances of C-14 ( 129.88),
C-9 ( 160.50), and C-13 ( 140.53). The data obtained
from a 'H-13C COLOC experiment also supported the
placement of the hydroxy group at C-9. Final proof of
the structure of 3 was obtained by methylation with
CH
2
N
2
to afford compound 1
9-methoxycanthin-6-one [coumpound1],
9-hydroxycanthin-6-one [coumpound3]
Compound 3, yellow needles, mp 285-286" (dec); uv max (MeOH)
(log E) 238 (sh, 4.20), 274 (4.20), 310 (4.05), 352 nm (4.15); (+
NaOH), 236 (sh, 4.25), 294 (4.15), 316 (sh, 4. 10), 355 (4.00),428
nm (3.90); ir v max (KBr) 3400-3600, 1700, 1663, 1653, 1636,
1570, 1559, 1457, 1355, 1320,1285, 1250, 1155, 1064 ,986,929,
850, 835, 635 cm-'; 'H nmr see Table 2; I3C nmr see Table 1; eims
(70 eV) m/z [M]+ 236 (100), 224 (10), 209 (8),2 08 (50), 197 (24),
179 (10), 150(58), 123 (22), 118 (5); hreims 236.0590 (calcd for
C
14
H
8
N
2
0
2
, 236.0585). When compound 3 (5 mg) was methylated
with CH
2
N
2
using the Diazald Kit (Aldrich, Milwaukee, Wisconsin), 4
mg of a product was obtained that was identical (mmp, 'H nmr, uv,
ir, ms) to compound 1.
Anda mungkin juga menyukai
- TerpenoidDokumen13 halamanTerpenoidnitaBelum ada peringkat
- Pinostrobin 1Dokumen5 halamanPinostrobin 1Rizky Ade PutraBelum ada peringkat
- Membuat Nanokoloid HSA untuk LimfosintigrafiDokumen14 halamanMembuat Nanokoloid HSA untuk LimfosintigrafiAfri YaniBelum ada peringkat
- Resume Artikel: Sintesis Derivatif Kalkon Novel Dari Miristikin Untuk Aktivitas Pencegahan Kanker KulitDokumen8 halamanResume Artikel: Sintesis Derivatif Kalkon Novel Dari Miristikin Untuk Aktivitas Pencegahan Kanker KulitRatu PuspaBelum ada peringkat
- Dua Isomer Flavonoid Terprenelasi Dari Daun Macaranga AleuritoidesDokumen8 halamanDua Isomer Flavonoid Terprenelasi Dari Daun Macaranga AleuritoidesDicky FeynmanBelum ada peringkat
- Radio Farmasi MakalahDokumen13 halamanRadio Farmasi MakalahInta TahakuBelum ada peringkat
- Review Jurnal Kimia Organik - (Puji Astuti A28226995)Dokumen5 halamanReview Jurnal Kimia Organik - (Puji Astuti A28226995)PUJI ASTUTIBelum ada peringkat
- PikacuDokumen30 halamanPikacuFika Wilda AnggraeniBelum ada peringkat
- Resume Jurnal Sintesis Pirazolin - Kelompok 13 S13ADokumen10 halamanResume Jurnal Sintesis Pirazolin - Kelompok 13 S13ARegina Sabela DiandaaBelum ada peringkat
- Jurnal BTNG Mdu Rni 06Dokumen5 halamanJurnal BTNG Mdu Rni 06Andyan RonaldoBelum ada peringkat
- Jurnal Kapsel SteroidDokumen4 halamanJurnal Kapsel SteroidMuhammad Yusi Anda RizkyBelum ada peringkat
- REVIEW JURNAL Fts 2 MirzaDokumen7 halamanREVIEW JURNAL Fts 2 Mirzaahmad mirzaBelum ada peringkat
- KATEKIN TEH HIJAUDokumen10 halamanKATEKIN TEH HIJAUAomineDaikiBelum ada peringkat
- Sintesis Dan Uji Toksisitas Senyawa 2',4'-Dikloro-4-Metoksikalkon - Jurnal 9Dokumen5 halamanSintesis Dan Uji Toksisitas Senyawa 2',4'-Dikloro-4-Metoksikalkon - Jurnal 9azalea titonBelum ada peringkat
- Studi Pendahuluan Kandungan Kimia Tumbuhan Paku Nephrolepis RadicansDokumen10 halamanStudi Pendahuluan Kandungan Kimia Tumbuhan Paku Nephrolepis RadicansNatalie CarReen RadjahBelum ada peringkat
- 4 74 1 PBDokumen5 halaman4 74 1 PBRangga AssidikBelum ada peringkat
- Morina 2Dokumen4 halamanMorina 2febrinBelum ada peringkat
- Tugas Makalah Desain Sintesis ObatDokumen11 halamanTugas Makalah Desain Sintesis ObatAnonymous Jhl1esaKBelum ada peringkat
- Analisis Air FormasiDokumen12 halamanAnalisis Air FormasiRizvanBelum ada peringkat
- KalkonDokumen5 halamanKalkonS Adhi NugrohoBelum ada peringkat
- Afk ResumeDokumen7 halamanAfk ResumeElisa Cynthia ArdarickaBelum ada peringkat
- Rangkuman Fitokimia AlkaloidDokumen21 halamanRangkuman Fitokimia AlkaloidFikaSetraRikantaraBelum ada peringkat
- Fika CantikDokumen19 halamanFika CantikFika Wilda AnggraeniBelum ada peringkat
- Ringkasan Farmasi BahariDokumen10 halamanRingkasan Farmasi BahariMery Kartika MaulidaBelum ada peringkat
- Deka Wahyu Kurniawan - 202001218P - Remed Kimor 2Dokumen3 halamanDeka Wahyu Kurniawan - 202001218P - Remed Kimor 2Deka WahyuBelum ada peringkat
- SintesisAzabikloDokumen8 halamanSintesisAzabikloMeilaDwiputriBelum ada peringkat
- Jurnal Kimia OrganikDokumen9 halamanJurnal Kimia OrganikB062 Desi IndahnfBelum ada peringkat
- Tugas Fitokimia Uas 1 - Georgia Nahak - Ii BDokumen4 halamanTugas Fitokimia Uas 1 - Georgia Nahak - Ii BGeorgia NahakBelum ada peringkat
- Steroid Mahkota DewaDokumen8 halamanSteroid Mahkota DewaRaisyIkrimahBelum ada peringkat
- Efek Terapi Nano-Enkapsulasi dan Nano-Emulsi Karvakrol pada Fibrosis HatiDokumen11 halamanEfek Terapi Nano-Enkapsulasi dan Nano-Emulsi Karvakrol pada Fibrosis HatiatinBelum ada peringkat
- Antitumor Alkaloid dari Biji Peganum HarmalaDokumen17 halamanAntitumor Alkaloid dari Biji Peganum HarmalanabilahBelum ada peringkat
- Sintesis Kalkon NaftalenDokumen4 halamanSintesis Kalkon Naftalenasa isiBelum ada peringkat
- Penetapan Kadar Flavonoid Total OrthosiphonDokumen8 halamanPenetapan Kadar Flavonoid Total OrthosiphonJackleen StephanieBelum ada peringkat
- Synthesis, Characterisation and Evaluation of Pt(II) and Au(I) Iminophosphine Complexes for Cancer TreatmentDokumen22 halamanSynthesis, Characterisation and Evaluation of Pt(II) and Au(I) Iminophosphine Complexes for Cancer TreatmentMahardika RahmaBelum ada peringkat
- Sintesis Derivat Calkon Novel Dari Myristicin Untuk Kegiatan Pencegahan Kanker KulitDokumen5 halamanSintesis Derivat Calkon Novel Dari Myristicin Untuk Kegiatan Pencegahan Kanker Kulitmonica septarina pBelum ada peringkat
- HPLCDokumen17 halamanHPLCWebinar UYBelum ada peringkat
- ISOLASI ALKALOID DARI DAUN BINAHONGDokumen26 halamanISOLASI ALKALOID DARI DAUN BINAHONGChachaBelum ada peringkat
- Ringkasan Wawasan Struktural Dari Peptida 9-ResiduDokumen9 halamanRingkasan Wawasan Struktural Dari Peptida 9-ResiduIndah Maria Tioday Lumban GaolBelum ada peringkat
- Fitokim HPLCDokumen6 halamanFitokim HPLCGorilaBelum ada peringkat
- 36 1 113 1 10 20180314 PDFDokumen4 halaman36 1 113 1 10 20180314 PDFRhiry'sriwaNelwan'sBelum ada peringkat
- KLOVANADIOLDokumen15 halamanKLOVANADIOLAgung SujatmikoBelum ada peringkat
- Hasil Pembahasan ArinDokumen5 halamanHasil Pembahasan ArinNurafinda 1703111046Belum ada peringkat
- Kimia Organik Handin Octalia A28226969 Teori 4Dokumen9 halamanKimia Organik Handin Octalia A28226969 Teori 4anacostantina blamenBelum ada peringkat
- Sintesis Derivat KalkonDokumen23 halamanSintesis Derivat KalkonRohaniBelum ada peringkat
- Senyawa Terpenoid Turunan Lupeol dari Kulit Batang PaliasaDokumen3 halamanSenyawa Terpenoid Turunan Lupeol dari Kulit Batang PaliasaLia Amanda Pulungan100% (1)
- Kimed 2 Hasriati F201901032 C1 FarmasiDokumen13 halamanKimed 2 Hasriati F201901032 C1 FarmasiHasriati C1 FarmasiBelum ada peringkat
- MetodeDokumen2 halamanMetodeYunita SupuBelum ada peringkat
- Laporan Fitokimia - Pertemuan V - Skrining Fitokimia Menggunakan KLTDokumen12 halamanLaporan Fitokimia - Pertemuan V - Skrining Fitokimia Menggunakan KLTgusti ngurahBelum ada peringkat
- Ekstraksi KecombrangDokumen12 halamanEkstraksi KecombrangSitti Munawarah IIBelum ada peringkat
- Laporan Praktikum KI2152 7 & 8Dokumen11 halamanLaporan Praktikum KI2152 7 & 8AvivSigitCahyonoBelum ada peringkat
- Review Jurnal Kimia Organik ObatDokumen8 halamanReview Jurnal Kimia Organik ObatVeronika Tiara Dewi AnandaBelum ada peringkat
- STEROID DARI Dysoxylum alliaceum AKTIF TERHADAP SEL MCF-7Dokumen4 halamanSTEROID DARI Dysoxylum alliaceum AKTIF TERHADAP SEL MCF-7Boy DullBelum ada peringkat
- ISOLASIDokumen28 halamanISOLASIFauzan PatawariBelum ada peringkat
- Aktivitas Anticoagulan Senyawa dari Tanaman Ainsliaea fragransDokumen13 halamanAktivitas Anticoagulan Senyawa dari Tanaman Ainsliaea fragransAlviana YuniantiBelum ada peringkat
- Jurnal Reading-MivacuriumDokumen11 halamanJurnal Reading-MivacuriumHilmy LabibiBelum ada peringkat
- REVIEW JURNAL MetodeKontrolkualitas Strontium Chloride 89SRCl2Dokumen3 halamanREVIEW JURNAL MetodeKontrolkualitas Strontium Chloride 89SRCl2Hilda Khairunnisa SholiqinBelum ada peringkat
- Tugas Review Elrysantri Rambu Tinggi Nalu A28226917Dokumen10 halamanTugas Review Elrysantri Rambu Tinggi Nalu A28226917ELRYSANTRI RAMBU TINGGI NALUBelum ada peringkat
- Uji Kmampuan Cao SBG An DLM Pengambilan Chrom DR Limbah Pnyamakan KulitDokumen3 halamanUji Kmampuan Cao SBG An DLM Pengambilan Chrom DR Limbah Pnyamakan KulitEndah Satria DewiBelum ada peringkat
- TabelPenulanganKolomDokumen18 halamanTabelPenulanganKolomadhimulyawanBelum ada peringkat
- Metode Kerja Sand Cone TestDokumen2 halamanMetode Kerja Sand Cone TestFraztya Hebby100% (1)
- BAB II Well CompletionDokumen33 halamanBAB II Well CompletionFraztya HebbyBelum ada peringkat
- Laporan Akhir Penyelidikan Tanah: Proyek White (WWTP Dan Biogas) Jl. Raya Kalijati, Subang Jawa BaratDokumen1 halamanLaporan Akhir Penyelidikan Tanah: Proyek White (WWTP Dan Biogas) Jl. Raya Kalijati, Subang Jawa BaratFraztya HebbyBelum ada peringkat
- Absensi LapanganDokumen1 halamanAbsensi LapanganFraztya HebbyBelum ada peringkat
- Membuat Grafik 3 DimensiDokumen5 halamanMembuat Grafik 3 DimensimutiaBelum ada peringkat
- JUDULDokumen2 halamanJUDULFraztya HebbyBelum ada peringkat
- Kurva SDokumen2 halamanKurva SFerdian DwiBelum ada peringkat
- Metode SeismikDokumen8 halamanMetode SeismikFajri MubarakBelum ada peringkat
- Kalibrasi - 0001 (97.245)Dokumen3 halamanKalibrasi - 0001 (97.245)Fraztya HebbyBelum ada peringkat
- FotoDokumen1 halamanFotoFraztya HebbyBelum ada peringkat
- Surat LamaranS1Dokumen1 halamanSurat LamaranS1ManuelNatalioBelum ada peringkat
- OPTIMIZED TITLEDokumen3 halamanOPTIMIZED TITLEFraztya HebbyBelum ada peringkat
- Table Data TanahDokumen7 halamanTable Data TanahJaka MilyadiBelum ada peringkat
- TA Bahar LengkapDokumen132 halamanTA Bahar LengkapFraztya HebbyBelum ada peringkat
- Tracking Februari SCB KGDokumen13 halamanTracking Februari SCB KGFraztya HebbyBelum ada peringkat
- PEMERIKSAAN KEKUATAN TANAH dengan SONDIR (DUTCH CONE PENETROMETERDokumen7 halamanPEMERIKSAAN KEKUATAN TANAH dengan SONDIR (DUTCH CONE PENETROMETERAnonymous KsiMP3OXyBelum ada peringkat
- 8839 CoverDokumen1 halaman8839 CoverFraztya HebbyBelum ada peringkat
- Metode Kerja Sand Cone TestDokumen2 halamanMetode Kerja Sand Cone TestFraztya Hebby100% (1)
- Biodata DiriDokumen2 halamanBiodata DiriFraztya HebbyBelum ada peringkat
- Yang DiprintDokumen20 halamanYang DiprintFraztya HebbyBelum ada peringkat
- COVERDokumen1 halamanCOVERFraztya HebbyBelum ada peringkat
- Yang DiprintDokumen20 halamanYang DiprintFraztya HebbyBelum ada peringkat
- LamDokumen1 halamanLamFraztya HebbyBelum ada peringkat
- COVERDokumen1 halamanCOVERFraztya HebbyBelum ada peringkat
- Pemeriksaan Kekuatan Tanah Dengan SondirDokumen19 halamanPemeriksaan Kekuatan Tanah Dengan SondirFraztya HebbyBelum ada peringkat
- Absensi LapanganDokumen1 halamanAbsensi LapanganFraztya HebbyBelum ada peringkat
- Absensi LapanganDokumen1 halamanAbsensi LapanganFraztya HebbyBelum ada peringkat
- Yang DiprintDokumen20 halamanYang DiprintFraztya HebbyBelum ada peringkat
- ReportDokumen1 halamanReportFraztya HebbyBelum ada peringkat