Ferrous Metals and Alloys
Diunggah oleh
LeonardDacaymat0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
109 tayangan44 halamanferrous metals and alloys
Hak Cipta
© © All Rights Reserved
Format Tersedia
PPTX, PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen Iniferrous metals and alloys
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PPTX, PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
109 tayangan44 halamanFerrous Metals and Alloys
Diunggah oleh
LeonardDacaymatferrous metals and alloys
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PPTX, PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 44
Presented by:
Benliro, Chin chin Pearl
Dacaymat, Leonard
Perez, Edmar
Vivas, Jean
Background on metals
The elements of all metals are found naturally
in the earth. However, they need to be extracted
and processed before they can be used for
manufacturing purposes. Because metals in their
most basic form are natural resources,. Metals
form part of the earths crust as metal ore. To
obtain useful metals, the metal ore is mined and
washed to remove other minerals and unwanted
materials. Iron ore is the basis for most steels. To
extract pure iron the iron ore is heated in a furnace
in a process known as smelting.
SMELTING
Categories of metals
Ferrous
metals
Non
Ferrous
metals
Iron
a soft, silvery metal that is the
fourth most abundant element in the
Earths crust. Pure iron is unobtainable
by smelting, but small amounts of
impurities can make iron many times
stronger than it exists in its pure form.
Ferrous Metals
Ferrous facts
Iron replaced bronze as
the principal metal by
1000 BC.
Early pots and pans made
from iron poisoned the
users!
Early steels were made by
adding carbon to iron as
it was melted over a
charcoal fire.
Ferrous metals:
contain iron
will corrode unless
protected
are attracted by a
magnet
are strong, rigid
and cheap.
Ferrous Metals
Ferrous metals are produced in larger quantities
than any other metallic material. Three factors account
for it:
availability of abundant raw materials combined with
economical extraction
ease of forming
their versatile mechanical and physical properties.
Ferrous metals have some disadvantages, includes:
poor corrosion resistance
Ferrous Metals & alloys
relatively high density
low electrical and thermal conductivities
Alloy
Sometimes ferrous and non-ferrous metals
require different properties in order to function better
in specific situations. Alloying metals involves mixing
two or more metals and other elements to improve
their properties.
Ferrous Metals and Alloys
Alloying metals can:
lower the melting point
alter thermal and electrical properties
make a material harder for cutting purposes
improve resistance to corrosion
help metal to flow better into a cast.
Ferrous Alloys
Ferrous Alloys
Ferrous Alloys
-Alloys containing Iron as the main element.
-The most important ferrous alloy system (Fe-C system)
-Alloys of this system can be further divided into steels
and cast irons.
-Then, all steels solidify into a single -Fe structure first
and then experience the complex eutectoid reaction.
Therefore, heat treatment processes, which alter the
eutectoid reaction, are vitally important for controlling
microstructure and properties of steels.
Ferrous Alloys
Another classification is made based on their
formability. If materials are hard to form, components
with these materials are fabricated by casting, thus
they are called cast alloys. If material can be
deformed, they are known as wrought alloys.
Materials are usually strengthened by two methods
cold work and heat treatment. Strengthening by heat
treatment involves either precipitation hardening or
martensitic transformation, both of which constitute
specific heat treating procedure. When a material can
not be strengthened by heat treatment, it is referred as
non-heat-treatable alloys.
Alloying Elements
Manganese
Nickel
Silicon
Chromium
Molybdenum
Vanadium
Tungsten
Improves hardness and toughness
Improves hardness
Improves toughness, shock resistance and
strength
Allows steel to be cut at high strength
Increases strength and toughness of heat-
treated steel
Increases strength and toughness in low
temperature
Increases resistance to wear and tear,
improves durability
Classification of terms
Steels
Steels are alloys of iron and carbon plus other
alloying elements. In steels, carbon present in atomic
form, and occupies interstitial sites of Fe
microstructure. Mechanical properties of steels are
very sensitive to carbon content. Hence, it is practical
to classify steels based on their carbon content.
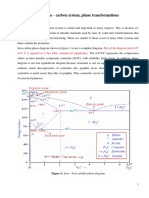
Steels - the ferrous alloys with less than 2.14% C
Cast Irons - the ferrous alloys with higher than 2.14% C
Steels
Steels are basically two kinds:
low-carbon steels (% wt of C < 0.3)
medium carbon steels (0.3 <% wt of C < 0.6)
high-carbon steels (% wt of C > 0.6)
1. (plain) Carbon steels
2. Alloy steels
low alloy (< 5 % total alloying element)
high alloy (> 5% total alloying element)
Carbon Steel
Carbon steel is a malleable, iron-based metal
containing less than 2% carbon (usually less than 1%),
small amounts of manganese, and other trace
elements. Steels can either be cast to shape or wrought
into various mill forms from which finished parts are
formed, machined, forged, stamped, or otherwise
shaped. Carbon steels are specified by chemical
composition, mechanical properties, method of
deoxidation, or thermal treatment.
Microscopic structure of Carbon steel
Low Carbon Steels
These are arguably produced in the greatest
quantities than other alloys. Carbon present in these
alloys is limited, and is not enough to strengthen these
materials by heat treatment; hence these alloys are
strengthened by cold work. Their microstructure
consists of ferrite and pearlite, and these alloys are
thus relatively soft, ductile combined with high
toughness. Hence these materials are easily
machinable and weldable. Typical applications of
these alloys include: structural shapes, tin cans,
automobile body components, buildings, etc.
Medium Carbon Steels
These are stronger than low carbon steels. However
these are of less ductile than low carbon steels. These alloys
can be heat treated to improve their strength. Usual heat
treatment cycle consists of austenitizing, quenching, and
tempering at suitable conditions to acquire required
hardness. They are often used in tempered condition. As
hardenability of these alloys is low, only thin sections can
be heat treated using very high quench rates. Ni, Cr and Mo
alloying additions improve their hardenability. Typical
applications include: railway tracks & wheels, gears, other
machine parts which may require good combination of
strength and toughness.
High Carbon Steels
These are strongest and hardest of carbon steels,
and of course their ductility is very limited. These are
heat treatable, and mostly used in hardened and
tempered conditions. They possess very high wear
resistance, and capable of holding sharp edges. Thus
these are used for tool application such as knives,
razors, hacksaw blades, etc. With addition of alloying
element like Cr, V, Mo, W which forms hard carbides
by reacting with carbon present, wear resistance of
high carbon steels can be improved considerably.
Alloy Steels
Steels that contain specified amounts of alloying
elements -- other than carbon and the commonly accepted
amounts of manganese, copper, silicon, sulfur, and
phosphorus -- are known as alloy steels. A steel is
considered to be an alloy when the maximum of the range
given for the content of alloying elements exceeds one or
more of these limits: 1.65% Mn, 0.60% Si, or 0.60% Cu; or
when a definite range or minimum amount of any of the
following elements is specified or required within the
limits recognized for constructional alloy steels: aluminum,
chromium (to 3.99%),
Alloy Steels
cobalt, columbium, molybdenum, nickel, titanium,
tungsten, vanadium, zirconium or other element
added to obtain an alloying effect. Technically, then,
tool and stainless steels are alloy steels.
High - Strength low - alloy
A special group of ferrous alloys with noticeable
amount of alloying additions are known as HSLA
(high-strength low-alloy) steels. Common alloying
elements are: Cu, V, Ni, W, Cr, Mo, etc. These alloys
can be strengthened by heat treatment, and yet the
same time they are ductile, formable. Typical
applications of these HSLA steels include: support
columns, bridges, pressure vessels.
Stainless steels
The name comes from their high resistance to
corrosion i.e. they are rust-less (stain-less). Steels are
made highly corrosion resistant by addition of special
alloying elements, especially a minimum of 12% Cr
along with Ni and Mo. Stainless steels are mainly three
kinds: ferritic & hardenable Cr steels, austenitic and
precipitation hardenable (martensitic, semi-
austenitic) steels. This classification is based on
prominent constituent of the microstructure. Typical
applications include cutlery, razor blades, surgical
knives, etc.
Stainless Steels: kinds
Ferritic stainless steel
Ferritic stainless steels are principally Fe-
Cr-C alloys with 12-14% Cr. They also contain
small additions of Mo, V, Nb, and Ni.
Austenitic Stainless Steel
Austenitic stainless steels usually contain 18% Cr
and 8% Ni in addition to other minor alloying
elements. Ni stabilizes the austenitic phase assisted by
C and N. Other alloying additions include Ti, Nb, Mo
(prevent weld decay), Mn and Cu (helps in stabilizing
austenite).
Stainless Steels: kinds
Martensitic stainless steels, typified by types
410/420/440, containing about 12Cr and 0.1C wt% as
the basic composition. They are not as corrosion
resistant as the other classes, but are extremely strong
and tough as well as highly machineable, and can be
hardened by heat treatment. They contain 11.5 to 18%
chromium and significant amounts of carbon. Some
grades include additional alloying elements in small
quantities.
Martensitic stainless steel
Stainless Steels: kinds
Duplex stainless steels are two-phase alloys based
on the Fe-Cr-Ni system. The specific advantages offered by
duplex stainless steels over conventional 300 series
stainless steels are strength (approximately twice that of
austenitic stainless steels), improved toughness and
ductility (compared to ferritic grades), and a superior
chloride SCC resistance and pitting resistance. The high
yield strength offers designers the use of thin-wall material
(which can lead to major reductions in weight) with
adequate pressure-containing and load-bearing capacity.
Duplex stainless steels have found widespread use in a
range of industries, particularly the oil and gas,
petrochemical, and pulp and paper industries.
Tool Steels
Tool steels are particular steels designed for being
made into tools. They are known for toughness,
resistance to abrasion, ability to hold a cutting edge,
and/or their resistance to deformation at high
temperatures. The three types of tool steel available
are cold work steels used in lower operating
temperature environments, hot work steels used at
elevated temperatures, and high speed steels able to
withstand even higher temperatures giving them the
ability to cut at higher speeds
Cast Iron
Cast iron is defined as an iron alloy with more than 2%
carbon as the main alloying element. In addition to carbon,
cast irons must also contain from 1 to 3% silicon which
combined with the carbon give them excellent castability.
Cast iron has a much lower melting temperature than steel
and is more fluid and less reactive with molding materials.
However, they do not have enough ductility to be rolled or
forged.
The precipitation of carbon (as graphite) during
solidification is the key to cast iron's distinctive properties.
The graphite provides excellent machinability (even at
wear-resisting hardness levels), damps vibration,
Cast Iron
and aids lubrication on wearing surfaces (even
under borderline lubrication conditions).
Tendency of cast irons to form graphite is usually
controlled by their composition and cooling rate.
Based on the form of carbon present, cast irons are
categorized as:
Gray cast Iron
White cast Iron
Nodular Cast Iron
Malleable cast irons
Gray Cast Iron
These alloys consists carbon in form graphite
flakes, which are surrounded by either ferrite or
pearlite. Because of presence of graphite, fractured
surface of these alloys look grayish, and so is the name
for them. Alloying addition of Si (1-3wt.%) is
responsible for decomposition of cementite, and also
high fluidity. Thus castings of intricate shapes can be
easily made. Due to graphite flakes, gray cast irons are
weak and brittle. However they possess good damping
properties, and thus typical applications include: base
structures, bed for heavy machines, etc. they also show
high resistance to wear.
Gray cast irons have good
mechanical properties in
compression, and are
particularly effective in
damping vibration. They are
commonly used for the bases
of heavy machinery for this
reason.
Grey cast irons also have low
cost, good wear resistance and
high fluidity with low
shrinkage during casting.
Fe 3.4wt%C 2.5wt%Si 0.01wt%P (Low P, high grade cast Iron)
White Cast Iron
When Si content is low (< 1%) in combination
with faster cooling rates, there is no time left for
cementite to get decomposed, thus most of the brittle
cementite retains. Because of presence of cementite,
fractured surface appear white, hence the name. They
are very brittle and extremely difficult to machine.
Hence their use is limited to wear resistant
applications such as rollers in rolling mills. Usually
white cast iron is heat treated to produce malleable
iron.
Fe-2.8wt%C-1.8wt%Si (White cast iron)
The cementite makes white cast
iron very hard and abrasion
resistant. It is commonly used for
rollers and wear resistant
surfaces. It is brittle and almost
impossible to machine.
Nodular (or Ductile) Cast Iron
Alloying additions are of prime importance in
producing these materials. Small additions of Mg / Ce
to the gray cast iron melt before casting can result in
graphite to form nodules or sphere-like particles.
Matrix surrounding these particles can be either ferrite
or pearlite depending on the heat treatment. These are
stronger and ductile than gray cast irons. Typical
applications include: pump bodies, crank shafts,
automotive components, etc.
Fe 3.4wt%C 2.5wt%Si 0.01wt%P 0.03wt%Mg pearlitic ductile or
nodular iron
Malleable Cast Iron
These formed after heat treating white cast iron.
Heat treatments involve heating the material up to
800-900 C, and keep it for long hours, before cooling
it to room temperature. High temperature incubation
causes cementite to decompose and form ferrite and
graphite. Thus these materials are stronger with
appreciable amount of ductility. Typical applications
include: railroad, connecting rods, marine and other
heavy-duty services.
Anda mungkin juga menyukai
- Types and Applications of MaterialsDokumen53 halamanTypes and Applications of MaterialsGeno Martinez100% (1)
- Material EngineeringDokumen46 halamanMaterial EngineeringBoaquin KhenBelum ada peringkat
- 2-BMCG2323 Manufaturing MaterialsDokumen91 halaman2-BMCG2323 Manufaturing Materialshemarubini96Belum ada peringkat
- Engineering MaterialsDokumen25 halamanEngineering MaterialsNichan CanilloBelum ada peringkat
- 11 Introduction To Engineering MaterialsDokumen20 halaman11 Introduction To Engineering MaterialsomkardashetwarBelum ada peringkat
- Recovery Recrystallization Grain GrowthDokumen15 halamanRecovery Recrystallization Grain Growthteju1996coolBelum ada peringkat
- Mechanical Properties PDFDokumen57 halamanMechanical Properties PDFvardhaBelum ada peringkat
- Mse Notes - Unit - 2Dokumen12 halamanMse Notes - Unit - 2337-ME- KIRTHAN DEVADIGABelum ada peringkat
- 12 - Fatigue of MetalsDokumen55 halaman12 - Fatigue of Metalsvoldemorts100% (1)
- Engineering Materials: Learning ObjectivesDokumen11 halamanEngineering Materials: Learning Objectives38Zeeshan ZameerBelum ada peringkat
- Material Science NotesDokumen11 halamanMaterial Science NotesRyan Ryan RyanBelum ada peringkat
- Strengthening Mechanisms PDFDokumen11 halamanStrengthening Mechanisms PDFSelva BabuBelum ada peringkat
- Metals and AlloysDokumen45 halamanMetals and AlloysAdhyt Tya PratamaBelum ada peringkat
- Plastic Deformation of MetalsDokumen42 halamanPlastic Deformation of MetalsNaresh DeshpandeBelum ada peringkat
- Introduction To Materials and Processes PDFDokumen0 halamanIntroduction To Materials and Processes PDFjayeshjpillaiBelum ada peringkat
- Extrusion and Its ApplicationDokumen28 halamanExtrusion and Its ApplicationLakhan GuptaBelum ada peringkat
- Welding ProblemsDokumen2 halamanWelding Problemsaksgupta24Belum ada peringkat
- Engineering MaterialsDokumen110 halamanEngineering MaterialsAthith D100% (1)
- Bonga University: Engineering Material (Meng2091)Dokumen19 halamanBonga University: Engineering Material (Meng2091)Mul'isaa JireenyaaBelum ada peringkat
- Metals.: Exercise 1: Make A List of All The Different Metals That You Know AboutDokumen29 halamanMetals.: Exercise 1: Make A List of All The Different Metals That You Know AboutDurgesh Patil100% (1)
- Metals NewDokumen36 halamanMetals NewAbenet GetachewBelum ada peringkat
- c1 Mechanical PropertiesDokumen46 halamanc1 Mechanical PropertiesHusnal TaufiqBelum ada peringkat
- Forming Basics For ClasssDokumen63 halamanForming Basics For ClasssMetalAnand ChelliahBelum ada peringkat
- Metals & Alloys - 2012Dokumen16 halamanMetals & Alloys - 2012Diong Kok HuiBelum ada peringkat
- 06 Strengthening MechanismsDokumen63 halaman06 Strengthening Mechanismspranavkumarparit100% (2)
- Solidification of MaterialDokumen28 halamanSolidification of MaterialNaman DaveBelum ada peringkat
- Material Science and Metallurgy: Unit I Structure of MaterialsDokumen127 halamanMaterial Science and Metallurgy: Unit I Structure of MaterialsViraj Babar100% (1)
- 2.b Poster - Ferrous Material and AlloyDokumen1 halaman2.b Poster - Ferrous Material and AlloyChristian Dave RoneBelum ada peringkat
- Forming V1Dokumen32 halamanForming V1Walid DamoniBelum ada peringkat
- Casting Lab 1 ReportDokumen8 halamanCasting Lab 1 Reportapi-253426167100% (1)
- Course Content: No. Title Slide NoDokumen54 halamanCourse Content: No. Title Slide NoDilip YadavBelum ada peringkat
- Assistant Professor Mechanical Department: Mr. G. Aravind ReddyDokumen67 halamanAssistant Professor Mechanical Department: Mr. G. Aravind ReddySai RamBelum ada peringkat
- Alloys: I) IntroductionDokumen12 halamanAlloys: I) IntroductionNikhil ShelarBelum ada peringkat
- Alloys and Types of SteelDokumen16 halamanAlloys and Types of SteelRajatBelum ada peringkat
- Casting: "Net Shape" or "Near-Net Shape" Process AdvantagesDokumen27 halamanCasting: "Net Shape" or "Near-Net Shape" Process AdvantagesnsbaruaoleBelum ada peringkat
- Special Casting ProcessDokumen9 halamanSpecial Casting ProcessChaitanya SadhanBelum ada peringkat
- Unit 2Dokumen50 halamanUnit 2Ravichandran GBelum ada peringkat
- Iron Carbon Phase DiagramDokumen4 halamanIron Carbon Phase DiagramMizanur RahmanBelum ada peringkat
- Engg - Materials - Effect of Alloying ElementDokumen22 halamanEngg - Materials - Effect of Alloying ElementSmruthi SuvarnaBelum ada peringkat
- MM PDF Ia1Dokumen109 halamanMM PDF Ia1M.41Mohd AnasBelum ada peringkat
- Chapter 9d FractureDokumen70 halamanChapter 9d FracturenaveenaBelum ada peringkat
- Extrusion of Metals: Mr. Jay Vora Faculty, School of Technology, PDPU, GandhinagarDokumen27 halamanExtrusion of Metals: Mr. Jay Vora Faculty, School of Technology, PDPU, GandhinagarAdityasinh DesaiBelum ada peringkat
- Ceramics Glasses Superconductors HODokumen4 halamanCeramics Glasses Superconductors HOMuhammad Raihan BalfasBelum ada peringkat
- Phase TransformationDokumen50 halamanPhase TransformationJitenderBelum ada peringkat
- Engineering MaterialsDokumen53 halamanEngineering MaterialsRAGINI PASUPULETIBelum ada peringkat
- Chapter 9 (Cleaning and Fettling of Castings), 2003Dokumen32 halamanChapter 9 (Cleaning and Fettling of Castings), 2003Desalegn DgaBelum ada peringkat
- Solidification, Phase Diagrams and Phase TransformationDokumen35 halamanSolidification, Phase Diagrams and Phase TransformationkrishnasaiBelum ada peringkat
- Dislocations and Strengthening Mechanisms: Module-6Dokumen29 halamanDislocations and Strengthening Mechanisms: Module-6Baskar ManiBelum ada peringkat
- REVGO Printable Flashcards (BT2) - Alvaro Cabanting JR For PrintDokumen101 halamanREVGO Printable Flashcards (BT2) - Alvaro Cabanting JR For Printjulyeeenx10969Belum ada peringkat
- Alloys and Classification of AlloysDokumen3 halamanAlloys and Classification of AlloysFaHeem KhAnBelum ada peringkat
- TEM Lecture CrystallineDokumen30 halamanTEM Lecture CrystallineSyed Abdul AhadBelum ada peringkat
- MZ FS Unit - 1Dokumen27 halamanMZ FS Unit - 1Jai KumarBelum ada peringkat
- Bme - Part 1Dokumen49 halamanBme - Part 1Sumanth ChallaBelum ada peringkat
- MODULE 5 Typical Engineering MaterialsDokumen39 halamanMODULE 5 Typical Engineering Materialssrinidhi kulkarniBelum ada peringkat
- Microstructure Study of Ferrous and Non Ferrous Alloys Under Various Compositions and Heat Treatment Conditions Lab ReportDokumen7 halamanMicrostructure Study of Ferrous and Non Ferrous Alloys Under Various Compositions and Heat Treatment Conditions Lab Reportzrro50% (4)
- Material Science: Prof. Satish V. KailasDokumen12 halamanMaterial Science: Prof. Satish V. KailasAlvin SmithBelum ada peringkat
- Imp NotesDokumen7 halamanImp Notes22102048Belum ada peringkat
- UNIT 2 PPT 1Dokumen42 halamanUNIT 2 PPT 1neha yarrapothuBelum ada peringkat
- 211 2aDokumen33 halaman211 2aMada ChohBelum ada peringkat
- Clase 25. Aleaciones FerrosasDokumen20 halamanClase 25. Aleaciones FerrosasbaparedesrBelum ada peringkat
- Boyer, Howard E. (Eds.) - Atlas of Creep and Stress-Rupture Curves-ASM International (1988)Dokumen599 halamanBoyer, Howard E. (Eds.) - Atlas of Creep and Stress-Rupture Curves-ASM International (1988)Dan VaderBelum ada peringkat
- Lecture 9 Erosion-CorrosionDokumen27 halamanLecture 9 Erosion-Corrosionprakush01975225403Belum ada peringkat
- Syllabus - B.E. Mechanical - 2009 RegulationDokumen161 halamanSyllabus - B.E. Mechanical - 2009 RegulationshivakumarBelum ada peringkat
- Iron Carbon Phase DiagramDokumen4 halamanIron Carbon Phase DiagramnaveedBelum ada peringkat
- ACO Systems Ductile Iron Brochure SmallDokumen28 halamanACO Systems Ductile Iron Brochure SmallilieoniciucBelum ada peringkat
- Materiales Serie XL PDFDokumen1 halamanMateriales Serie XL PDFcarolina PortocarreroBelum ada peringkat
- Cast Iron: Gray Iron Ferrous Alloys Eutectic CarbideDokumen12 halamanCast Iron: Gray Iron Ferrous Alloys Eutectic CarbideMANOJ MBelum ada peringkat
- Selection of Materials For Engineering ApplicationsDokumen151 halamanSelection of Materials For Engineering ApplicationsCharitha Ranwala100% (1)
- Nitrogen Fissures Defects in Iron Castings: Back ToDokumen2 halamanNitrogen Fissures Defects in Iron Castings: Back ToJustin DixonBelum ada peringkat
- Ampco Fristam Replacement Parts GuideDokumen2 halamanAmpco Fristam Replacement Parts GuideИбрагим НурмамедовBelum ada peringkat
- All Metals - Glossary of Metallurgical and Materials Testing TermsDokumen22 halamanAll Metals - Glossary of Metallurgical and Materials Testing TermshjoutipBelum ada peringkat
- Corrosion Behaviour of CIDokumen1 halamanCorrosion Behaviour of CIBaher ElsheikhBelum ada peringkat
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDokumen13 halamanChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUBelum ada peringkat
- Diop, Cheikh Anta - A Origem Africana Da Civilização - Mito Ou RealidadeDokumen230 halamanDiop, Cheikh Anta - A Origem Africana Da Civilização - Mito Ou RealidadeJosé Renato Teixeira100% (1)
- Electrum MagicumDokumen5 halamanElectrum MagicumVitor Campos100% (1)
- Chapter 7 33Dokumen9 halamanChapter 7 33ayushBelum ada peringkat
- Cast IronsDokumen4 halamanCast IronsMohamad FaizulBelum ada peringkat
- Elliott Yr SpecificationsDokumen8 halamanElliott Yr SpecificationsOscar Barajas BBelum ada peringkat
- A834Dokumen4 halamanA834mithileshBelum ada peringkat
- Utp 83 FNDokumen1 halamanUtp 83 FNSun SunBelum ada peringkat
- Use of Silicon Carbide in Induction in Induction FurnaceDokumen2 halamanUse of Silicon Carbide in Induction in Induction FurnacemkraijadaBelum ada peringkat
- ORM Catalogue No. 886a Sep2020 CompressedDokumen61 halamanORM Catalogue No. 886a Sep2020 CompressedMetal deptBelum ada peringkat
- Workshop Science and Calculation QuestionsDokumen19 halamanWorkshop Science and Calculation QuestionsRaz aryan100% (1)
- Operational Information The Two Stroke Crosshead Engine The Cylinder LinerDokumen14 halamanOperational Information The Two Stroke Crosshead Engine The Cylinder LinerAayush AgrawalBelum ada peringkat
- Magna 770Dokumen20 halamanMagna 770கோகுல் இராBelum ada peringkat
- Plumbing Theory NotesDokumen133 halamanPlumbing Theory NotesAustine OtienoBelum ada peringkat
- Carbide GradesDokumen51 halamanCarbide GradesRicardo BravoBelum ada peringkat
- Effect of Chill On Ni Cast Iron PDFDokumen18 halamanEffect of Chill On Ni Cast Iron PDFsachinguptachdBelum ada peringkat
- CAST IRON AT SUB ZERO TEMPERATURES - Pump Engineering - Eng-TipsDokumen5 halamanCAST IRON AT SUB ZERO TEMPERATURES - Pump Engineering - Eng-Tipsvamsi patnalaBelum ada peringkat
- Bridge Research Assignment 1Dokumen4 halamanBridge Research Assignment 1RuthBelum ada peringkat