Lecture 2: Vectors, cDNA Libraries, & Plasmid Minipreps
Diunggah oleh
Rachu RajDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Lecture 2: Vectors, cDNA Libraries, & Plasmid Minipreps
Diunggah oleh
Rachu RajHak Cipta:
Format Tersedia
Lecture 2: Vectors, cDNA Libraries, & Plasmid
Minipreps
Introduction to Vectors
In order to study a DNA fragment (e.g., a gene), it
needs to be amplified and eventually purified.
These tasks are accomplished by cloning the DNA into
a vector.
A vector is generally a small, circular DNA molecule that
replicates inside a bacterium such as Escherichia coli
(can be a virus).
p. 2-1
Cloning Scheme
Digest Ligate Amplify and Prep
p. 2-1
Vector Types
There are three commonly used types of vectors:
1) plasmid vectors (e.g., pUC plasmids);
2) bacteriophage vectors (e.g., phage ); and
3) phagemid vectors (e.g., pBlueScript
TM
).
Each has a different use, and there are many derivatives of
these basic building blocks. In this course, you will be using
plasmid vectors.
p. 2-1
Plasmids
Circular DNA molecules found in bacteria
Replicated by the hosts machinery
independently of the genome. This is
accomplished by a sequence on the plasmid
called ori, for origin of replication.
Some plasmids are present in E. coli at 200-
500 copies/cell
p. 2-1
Plasmids also contain selectable markers.
Genes encoding proteins which provide a selection for
rapidly and easily finding bacteria containing the plasmid.
Provide resistance to an antibiotic (ampicillin, kanamycin,
tetracycline, chloramphenicol, etc.).
Thus, bacteria will grow on medium containing these
antibiotics only if the bacteria contain a plasmid with the
appropriate selectable marker.
Plasmid Engineering
p. 2-2
Transforming plasmids
into bacteria
p. 2-2
Safety Features
Modern cloning plasmids have been engineered so that they are
incapable of transfer between bacterial cells
Provide a level of biological containment.
Naturally occurring plasmids with their associated drug resistance genes
are responsible for the recent rise in antibiotic-resistant bacteria plaguing
modern medicine.
p. 2-3
Screening for Inserts
p. 2-3
Plasmid cloning vector
pDONR222
p. 2-4
Cloning a DNA fragment into pDONR222
Lethal
Viable
Transform Transform
p. 2-4
DNA Libraries
DNA library - a random collection of DNA fragments
from an organism cloned into a vector
Ideally contains at least one copy of every DNA
sequence.
Easily maintained in the laboratory
Can be manipulated in various ways to facilitate the
isolation of a DNA fragment of interest to a scientist.
Numerous types of libraries exist for various
organisms - Genomic and cDNA.
p. 2-5
Construction and analysis
of a genomic DNA library
p. 2-5
Construction of a
cDNA library
p. 2-6
Differences between a genomic and cDNA library
p. 2-7
Genomic Library
Promoters
Introns
Intergenic
Non-expressed genes
cDNA Library
Expressed genes
Transcription start sites
Open reading frames (ORFs)
Splice points
Purification of mRNA
p. 2-8
Collect and grind up animals
in mild denaturing solution
Spin out debris (Tissue,
membranes, etc)
Treat with DNAse
(removes DNA)
Treat with Phenol
(removes protein)
Synthesis of cDNA from mRNA
p. 2-8
Cloning a DNA fragment into pDONR222
Lethal
Viable
Transform Transform
p. 2-4
Preparing Plasmid DNA
In order to use a vector for cloning, sequencing, etc., it is
necessary to isolate the vector in a highly purified form.
Routinely done by most labs.
Many companies now sell kits which provide all the
solutions necessary for preparing DNA.
Based on similar procedures
p. 2-10
Essential components of minipreps
Gentle lysis step to break open the cells and
release the plasmid DNA into solution.
Cell debris and chromosomal DNA of the
bacteria is pelleted during the centrifugation.
Plasmid DNA remains behind in the clear
nonpelleted fraction (the nonpelleted solution left
after centrifugation is known as the
supernatant).
Subsequent steps are then performed on the
supernatant to remove contaminating RNA and
proteins from the plasmid DNA.
p. 2-10
Grow an overnight (ON) culture of the desired bacteria in
2 ml of LB medium containing the appropriate antibiotic
for plasmid selection. Incubate the cultures at 37C with
vigorous shaking.
1. Grow the bacteria
p. 2-11
T20AV12.09
Naming your clones
Year Your initials
Group # Clone #
Day
T (Teusday), W (Wednesday), H (Thursday)
2a. Transfer the cells to a tube and
centrifuge
Transfer 1.5 ml of the culture to a
microfuge tube and pellet the cells
for 1 minute at full speed (12,000
rpm) in the microcentrifuge.
First tap or gently vortex the glass
culture tube to resuspend the cells
which have settled. The culture can be
transferred to the microfuge tube by
pouring.
p. 2-11
2b. Remove the supernatant
Remove the growth medium (supernatant or
sup) by aspiration or by using the P-1000.
Leave the bacterial pellet as dry as possible so
that additional solutions are not diluted.
p. 2-11 (fig not shown)
3. Resuspend the cell pellet
Resuspend the bacterial pellet in 250 l of
Buffer PI by vigorous vortexing.
Add 150 ml of PI, cap the tube, and vortex on the
highest setting (pipetman can be used). Look very
closely for any undispersed pellet before proceeding
to the next step. It is essential that the pellet be
completely dispersed.
PI contains two essential components: Tris and
EDTA.
Tris is used to buffer the pH of the cell suspension.
EDTA is a chemical that chelates divalent cations
(ions with charges of +2) in the suspension, such as
Mg
++
. This helps break down the cell membrane
and inactivate intracellular enzymes.
p. 2-11
4. Add Solution P2
Add 250 l of Solution P2 (0.2 N
NaOH, 1%SDS), mix gently 4-6
times. Do not vortex!! This will
shear the DNA and contaminate
your DNA preps.
Denatures protein, DNA, RNA,
membranes. During this step a
viscous bacterial lysate forms (the
cells lyse).
p. 2-12
5. Add Solution N3
Add 350 l of Solution N3 (3 M
KOAc, pH 4.8). Mix gently 4-6
times. Do not vortex.
P3 neutralizes cell suspension. A
white precipitate consisting of
aggregated chromosomal DNA,
RNA and cell debris and SDS will
form.
Plasmids will renature
p. 2-12
6. Centrifuge cell debris
Centrifuge for 10 minutes at full
speed in the microcentrifuge.
A white pellet will form on the
bottom and side of the tube after
centrifugation.
p. 2-13
7. Transfer sup. (DNA) to spin column.
Using a P-1000 set at 600ul, transfer the supernatant
to the appropriately labeled spin column which has
been inserted into the 2 ml microcentrifuge tube.
p. 2-13 (Fig not shown)
8. Centrifuge the spin column
Centrifuge for 1 minute at full
speed, and drain the flow-
through from the collection
tube.
p. 2-13
9. Wash the column with PE
Add 750 ul of Wash buffer PE to
the spin column contained in
the 2 ml Collection Tube,
centrifuge at full speed for 1
minute, and drain the
flowthrough.
This buffer helps to further remove
any nucleases that may have co-
purified with the DNA. Remove the
liquid that has passed through the
column in the same way as
performed in Step 9.
p. 2-13
10. Spin the column to remove PE
Place the spin column in a fresh 1.7 ml
microcentrifuge tube (with lid cut off)
and centrifuge again for 1 minute at full
speed to remove any residual wash
solution that might still be in the
column.
Any residual wash solution must be
removed because the ethanol contained in
this solution may interfere with further DNA
manipulations. It is normal to remove a
small amount of liquid from the column at
this step, however if a significant amount of
solution (50-100 ul or greater) is found in
the collection tube, repeat this step.
p. 2-14 (Figure not shown)
11. Elute the DNA with EB
Place the spin column into an
appropriately labeled 1.7 ml
microcentrifuge tube and add 50 ul of
EB buffer to the column. Centrifuge
at full speed for 1 minute.
Elutes the plasmid DNA from the
column and collects in the
microcentrifuge tube.
p. 2-14
12. Store your DNA
Remove the spin column from the labeled
1.7 ml microcentrifuge tube and close the
lid on the tube tightly.
Store the miniprep DNA in your
freezer box (-20C).
p. 2-14
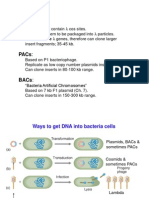
Other types of vectors
1. Phage Vectors
p. 2-15
2. Phagemids
Have plasmid and phage components
F1 Phage origin of
replication for
making single strand
DNA
p. 2-15
3. Cosmids - Plasmids with phage packaging sites
p. 2-16
4. YACs - Yeast Artificial Chromosomes
p. 2-17
Vector Insert Size
Vector Type Cloned DNA (Kb)
Plasmid 20
Phage 25
Cosmid 45
BAC 300
YAC 1000
p. 2-17
Anda mungkin juga menyukai
- Biochemistry Notes NbdeDokumen91 halamanBiochemistry Notes NbdeVivek Cheruvathoor100% (1)
- Genomic Dna ManualDokumen7 halamanGenomic Dna ManualZafran KhanBelum ada peringkat
- 3bsmt1 Bobier, Ashwelldonne Molbio PCRDokumen9 halaman3bsmt1 Bobier, Ashwelldonne Molbio PCRAshwell Donne BobierBelum ada peringkat
- Zheng Hong 郑 红 Department of Medical Genetics & Cell BiologyDokumen63 halamanZheng Hong 郑 红 Department of Medical Genetics & Cell BiologyinakiBelum ada peringkat
- Isolation of Bacterial Plasmid DNA (Compatibility Mode)Dokumen18 halamanIsolation of Bacterial Plasmid DNA (Compatibility Mode)Khandoker Faisal100% (1)
- ASH SAP, Seventh Edition, 2019 PDFDokumen798 halamanASH SAP, Seventh Edition, 2019 PDFDave Macedo100% (9)
- Acfrogasphivh-Xtfhfpduf9lboveax C F2-2hhardm - Fuja64eufnscgzylrzuq6buogp7smhqrx5gxz9es70bkuih2 TJ Ws6cvoavzh 4 Kwdqenvbguq6g5dfpy5ig2ou3yw58utcqdtekDokumen3 halamanAcfrogasphivh-Xtfhfpduf9lboveax C F2-2hhardm - Fuja64eufnscgzylrzuq6buogp7smhqrx5gxz9es70bkuih2 TJ Ws6cvoavzh 4 Kwdqenvbguq6g5dfpy5ig2ou3yw58utcqdtekEVEisFunBelum ada peringkat
- Life Sciences Fundamentals and Practice - IIDokumen161 halamanLife Sciences Fundamentals and Practice - IIPathifnder Publication81% (21)
- Lab Manual Molecular BiologyDokumen19 halamanLab Manual Molecular BiologyLockerLingBelum ada peringkat
- Lab Report Exp 2 Plasmid ExtractionDokumen8 halamanLab Report Exp 2 Plasmid ExtractionAmeera ShahirahBelum ada peringkat
- Machine Elements Life and Design: Boris M. Klebanov David M. Barlam Frederic E. NystromDokumen5 halamanMachine Elements Life and Design: Boris M. Klebanov David M. Barlam Frederic E. NystromRachu Raj100% (1)
- Mini PrepDokumen6 halamanMini PrepWilson GomargaBelum ada peringkat
- CH1801 C5 Plasmid IsolationDokumen7 halamanCH1801 C5 Plasmid IsolationTanisha ChowdharyBelum ada peringkat
- Purification of Plasmid DNA: MaterialsDokumen3 halamanPurification of Plasmid DNA: MaterialsTham Su MingBelum ada peringkat
- C5 Plasmid Isolation Formal ReportDokumen9 halamanC5 Plasmid Isolation Formal ReportTanisha ChowdharyBelum ada peringkat
- Practical3A 3B RDT 2016-17Dokumen5 halamanPractical3A 3B RDT 2016-17Carina JLBelum ada peringkat
- POB Lab ManualDokumen11 halamanPOB Lab ManualWEY LOON LIMBelum ada peringkat
- Plasmid LabDokumen10 halamanPlasmid LabAhmed J AlhindaweBelum ada peringkat
- Dna07 2Dokumen2 halamanDna07 2Sabesan TBelum ada peringkat
- PMT 324 Molecular Biology of The GeneDokumen6 halamanPMT 324 Molecular Biology of The GeneBlameless ArikoBelum ada peringkat
- Isolation and Purification of Total Genomic DNA From E. ColiDokumen6 halamanIsolation and Purification of Total Genomic DNA From E. ColiscribdsadhanaBelum ada peringkat
- Dna Isolation From e Coli ProtocolDokumen5 halamanDna Isolation From e Coli ProtocolMegh Raj BhattBelum ada peringkat
- Collective Lab Report 2 MolecularDokumen12 halamanCollective Lab Report 2 Molecularmariam farragBelum ada peringkat
- Report 3 and 4Dokumen11 halamanReport 3 and 4tinanassar2004Belum ada peringkat
- Expression of Recombinant Proteins in The Methylotrophic Yeast Pichia PastorisDokumen5 halamanExpression of Recombinant Proteins in The Methylotrophic Yeast Pichia PastorisAlexis TorresBelum ada peringkat
- Plasmid Isolation Using Alkaline Lysis (Exp 3, CSS451)Dokumen4 halamanPlasmid Isolation Using Alkaline Lysis (Exp 3, CSS451)Shubham GajraniBelum ada peringkat
- Competent Cells and TransformationDokumen22 halamanCompetent Cells and TransformationCarinaJongLeeBelum ada peringkat
- Molecular Biology - Amity University RajasthanDokumen13 halamanMolecular Biology - Amity University Rajasthanabash_u1Belum ada peringkat
- Protocol AxyPrep Blood Genomic DNA MiniprepDokumen5 halamanProtocol AxyPrep Blood Genomic DNA MiniprepRodolfo Bucarey UrreaBelum ada peringkat
- Nanocin - STANDARD & RAPID PROTOCOL - Plasmid Transfection - HEK293T Cells PDFDokumen3 halamanNanocin - STANDARD & RAPID PROTOCOL - Plasmid Transfection - HEK293T Cells PDFAnonymous fxxWImXUB9Belum ada peringkat
- Test 10Dokumen4 halamanTest 10CiprianBelum ada peringkat
- DNA Lab 1Dokumen4 halamanDNA Lab 1Abdul Mueez LoneBelum ada peringkat
- Mol 114 Lab HandoutDokumen6 halamanMol 114 Lab Handouthmnjh7crcfBelum ada peringkat
- Expt-6 (Isolation of Plasmid DNA)Dokumen4 halamanExpt-6 (Isolation of Plasmid DNA)Anne Nirmani RodrigoBelum ada peringkat
- Mammalian Genomic DNA Miniprep KitsDokumen6 halamanMammalian Genomic DNA Miniprep KitsRajan RawalBelum ada peringkat
- Lab Report 1: Dna Extraction From Peripheral Blood Mononuclear Cells (PBMC)Dokumen7 halamanLab Report 1: Dna Extraction From Peripheral Blood Mononuclear Cells (PBMC)Nida RidzuanBelum ada peringkat
- DVM 3rd M3 (Micro 403) VerifiedDokumen31 halamanDVM 3rd M3 (Micro 403) VerifiedHamza ShoukatBelum ada peringkat
- RBCL Competent Cell (E. Coli) PreparationDokumen6 halamanRBCL Competent Cell (E. Coli) Preparationme_dayakarBelum ada peringkat
- Experiment 5 &6 "Plasmid Transformation" "Isolation of Plasmid Dna From Bacterial Cells"Dokumen9 halamanExperiment 5 &6 "Plasmid Transformation" "Isolation of Plasmid Dna From Bacterial Cells"alperlengerBelum ada peringkat
- Genetic Lab NotebookDokumen11 halamanGenetic Lab NotebookKevin Chen100% (1)
- Transformationandtransfectionfinal 180609201420 PDFDokumen35 halamanTransformationandtransfectionfinal 180609201420 PDFDarshan MarjadiBelum ada peringkat
- Miniprep Plasmid Dna Isolation With The Boiling MethodDokumen5 halamanMiniprep Plasmid Dna Isolation With The Boiling MethodEbruAkharmanBelum ada peringkat
- Ankur Bhaumik 18895 UB201 Molecula Biology Lab ReportsDokumen19 halamanAnkur Bhaumik 18895 UB201 Molecula Biology Lab ReportsAnkur BhaumikBelum ada peringkat
- The Inoue Method For Preparation and Transformation of Competent E. Coli Ultra Competent CellsDokumen8 halamanThe Inoue Method For Preparation and Transformation of Competent E. Coli Ultra Competent Cellszeyneptozan136Belum ada peringkat
- SiO2 MethodDokumen8 halamanSiO2 MethodVageeshbabu HanurBelum ada peringkat
- Bacterial Dna Extraction EngDokumen4 halamanBacterial Dna Extraction EngLindokuhle NdzumoBelum ada peringkat
- Extraction of High Molecular Weight DNA From Eukaryotic Cells Molecular Biology Lab #7Dokumen4 halamanExtraction of High Molecular Weight DNA From Eukaryotic Cells Molecular Biology Lab #7Mahnoor ArshadBelum ada peringkat
- DNA MiniprepDokumen3 halamanDNA Miniprepifti007Belum ada peringkat
- Izolare ADN Bacterii 2Dokumen2 halamanIzolare ADN Bacterii 2Ionela ZubcoBelum ada peringkat
- Plasmid DNA Isolation PDFDokumen3 halamanPlasmid DNA Isolation PDF9001 Trisha BakshiBBT2Belum ada peringkat
- Artifact For Laboratory TechniquesDokumen17 halamanArtifact For Laboratory Techniquesapi-694576377Belum ada peringkat
- Plasmidextraction 2002Dokumen2 halamanPlasmidextraction 2002Sahithi KotcherlakotaBelum ada peringkat
- Gene Tagging - Bio WikiDokumen11 halamanGene Tagging - Bio WikiAkinsete Olaolu100% (1)
- Plasmid ExtractionDokumen7 halamanPlasmid ExtractionaraneyaBelum ada peringkat
- mİNİPREPDokumen12 halamanmİNİPREPEylül Nur ÇakırBelum ada peringkat
- 08 DNAExtractionDokumen6 halaman08 DNAExtraction22I87O54 - Bùi Duy Đăng KhoaBelum ada peringkat
- Genomic DNA Plug KitDokumen12 halamanGenomic DNA Plug KitsrijitkhanBelum ada peringkat
- Gene TransformationDokumen13 halamanGene TransformationFelix Ivander SalimBelum ada peringkat
- Ex. 5 DNA Extraction PDFDokumen3 halamanEx. 5 DNA Extraction PDFAlyssa Pauline PalacioBelum ada peringkat
- DNA ExtractionDokumen7 halamanDNA ExtractionyoshiBelum ada peringkat
- Roche MidiprepDokumen7 halamanRoche MidiprepBee CarbonellBelum ada peringkat
- Lab Practical 3: DNA EXTRACTIONDokumen2 halamanLab Practical 3: DNA EXTRACTIONCaroline H DavidBelum ada peringkat
- 5BICH003W - Lab Practical - 1 Protocol - 2023Dokumen13 halaman5BICH003W - Lab Practical - 1 Protocol - 2023aishatahliil03Belum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- A Strategy For Performance ExcellenceDokumen32 halamanA Strategy For Performance ExcellencebhogbhogaBelum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- New Doc 10Dokumen1 halamanNew Doc 10Rachu RajBelum ada peringkat
- A Strategy For Performance ExcellenceDokumen32 halamanA Strategy For Performance ExcellencebhogbhogaBelum ada peringkat
- Make The Number.: SmallestDokumen10 halamanMake The Number.: SmallestRachu RajBelum ada peringkat
- 5 SDokumen15 halaman5 SViswanathan SrkBelum ada peringkat
- 5 SDokumen15 halaman5 SViswanathan SrkBelum ada peringkat
- B Tech Scheme Revised Syllabus Equivalent Subjects-1Dokumen23 halamanB Tech Scheme Revised Syllabus Equivalent Subjects-1Rachu RajBelum ada peringkat
- Moleculkbnoknlo Ughiufvbik IouhgDokumen26 halamanMoleculkbnoknlo Ughiufvbik IouhgRachu RajBelum ada peringkat
- CosmidsDokumen8 halamanCosmidsRachu RajBelum ada peringkat
- PG Ccss2407ljbhDokumen11 halamanPG Ccss2407ljbhRachu RajBelum ada peringkat
- CccutilDokumen1 halamanCccutilJose Enrique Flores GonzalezBelum ada peringkat
- Yeast Shuttle VectorsDokumen5 halamanYeast Shuttle VectorsraphiaBelum ada peringkat
- (Office Copy. Please Attach With The Application) : Page 1/1Dokumen1 halaman(Office Copy. Please Attach With The Application) : Page 1/1Rachu RajBelum ada peringkat
- PG Ccss2407ljbhDokumen11 halamanPG Ccss2407ljbhRachu RajBelum ada peringkat
- IijjkDokumen2 halamanIijjkRachu RajBelum ada peringkat
- Yeast Shuttle VectorsDokumen5 halamanYeast Shuttle VectorsraphiaBelum ada peringkat
- File WWWDokumen1 halamanFile WWWRachu RajBelum ada peringkat
- CosmidsDokumen8 halamanCosmidsRachu RajBelum ada peringkat
- IijjkDokumen2 halamanIijjkRachu RajBelum ada peringkat
- (Office Copy. Please Attach With The Application) : Page 1/1Dokumen1 halaman(Office Copy. Please Attach With The Application) : Page 1/1Rachu RajBelum ada peringkat
- First: Elevation Section On BBDokumen1 halamanFirst: Elevation Section On BBBoppineti Naga RajuBelum ada peringkat
- Alumni PDF HubDokumen14 halamanAlumni PDF HubasanyogBelum ada peringkat
- Department of Education: Individual Output On Unpacked MelcsDokumen1 halamanDepartment of Education: Individual Output On Unpacked MelcsBalagtas VinaBelum ada peringkat
- B InggrisDokumen8 halamanB InggrisTrisna EniBelum ada peringkat
- Growth & Diffferentiation in Tissue CultureDokumen43 halamanGrowth & Diffferentiation in Tissue CulturelordniklausBelum ada peringkat
- BK 15.7 CheDokumen62 halamanBK 15.7 CheVenkata RamananBelum ada peringkat
- Homework 1 &2 Biology IUDokumen5 halamanHomework 1 &2 Biology IUVanessa NguyenBelum ada peringkat
- Cornell Notes CH 7Dokumen2 halamanCornell Notes CH 7Aaron WongBelum ada peringkat
- Molecular BiologyDokumen11 halamanMolecular BiologyAsish GeiorgeBelum ada peringkat
- Chapter 13 Section 1-2 Study StationsDokumen9 halamanChapter 13 Section 1-2 Study StationsBao HoangBelum ada peringkat
- 2019 NAFEAHajin KimDokumen19 halaman2019 NAFEAHajin KimJudicaëlBelum ada peringkat
- Pharmaceutical Business Management: Group 9Dokumen25 halamanPharmaceutical Business Management: Group 9Dhanashree TeliBelum ada peringkat
- The Wd40 Protein Bamb Mediates Coupling of Bam Complexes Into Assembly Precincts in The Bacterial Outer MembraneDokumen14 halamanThe Wd40 Protein Bamb Mediates Coupling of Bam Complexes Into Assembly Precincts in The Bacterial Outer MembraneAMMAR RAZABelum ada peringkat
- An Emerging Coronavirus Causing Pneumonia Outbreak in Wuhan China Calling For Developing Therapeutic and Prophylactic Strategies PDFDokumen4 halamanAn Emerging Coronavirus Causing Pneumonia Outbreak in Wuhan China Calling For Developing Therapeutic and Prophylactic Strategies PDFGemmaBelum ada peringkat
- Worksheet 1 - NCERT BasedDokumen4 halamanWorksheet 1 - NCERT BasedSonia GuptaBelum ada peringkat
- BiostatisticsDokumen4 halamanBiostatisticsmuinbossBelum ada peringkat
- B.seshu BabuDokumen3 halamanB.seshu BabuDerek FletcherBelum ada peringkat
- Cbse Biology INBO Olympiad Question Papers 2018Dokumen34 halamanCbse Biology INBO Olympiad Question Papers 2018Sri Jatin NammiBelum ada peringkat
- Research Method Proposal On Teak Tissue CultureDokumen8 halamanResearch Method Proposal On Teak Tissue CultureAmalina Mohamed SooudinBelum ada peringkat
- Nda and AndaDokumen21 halamanNda and AndaPavan KumarBelum ada peringkat
- Teori SelDokumen33 halamanTeori SelHafidah RakhmatinaBelum ada peringkat
- Polyplus Transfection® Life Science Research and Non-Viral In-Vivo Transfection ReagentsDokumen6 halamanPolyplus Transfection® Life Science Research and Non-Viral In-Vivo Transfection ReagentsDiego GarridoBelum ada peringkat
- Maccura Laboratories New Test Slides Rene PDFDokumen4 halamanMaccura Laboratories New Test Slides Rene PDFRicardo NinaBelum ada peringkat
- Clinical Research Scientist in Washington DC Resume Catherine KibirigeDokumen2 halamanClinical Research Scientist in Washington DC Resume Catherine KibirigeCatherineKibirigeBelum ada peringkat
- An Introduction To Fermentation ProcessesDokumen18 halamanAn Introduction To Fermentation ProcessesPhú NguyễnBelum ada peringkat
- BiologyDokumen2 halamanBiologyapi-313052886Belum ada peringkat