Acid Bronsted
Diunggah oleh
Branimir ZivanovicHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Acid Bronsted
Diunggah oleh
Branimir ZivanovicHak Cipta:
Format Tersedia
Properties of acids and bases
Get 8 test tubes. Rinse all tubes well with water.
Add acid to four tubes, base to the other four.
Touch a drop of base to your finger. Record
the feel in the chart (on the next slide). Wash
your hands with water. Repeat for acid.
Use a stirring rod, add base to the litmus and
pH papers (for pH paper use a colour key to
find a number). Record results. Repeat for acid.
Into the four base tubes add: a) two drops of
phenolphthalein, b) 2 drops of bromothymol, c)
a piece of Mg, d) a small scoop of baking soda.
Record results. Repeat for acid.
Clean up (wash tubes, pH/litmus paper in trash).
Bubbles NR Baking soda
Bubbles NR Magnesium
*Yellow *Blue Bromothymol
*Cloudy/
white
*Pink Phenolphthalein
Red Blue Litmus (blue or red)
1 14 pH (# from the key)
Not slippery Slippery
Feel (choose slippery
or not slippery)
Sour Bitter Taste
HCl(aq) NaOH(aq)
Observations
*Usually, but not always
pH
There are many ways to consider acids and
bases. One of these is pH. Read pg. 368-70.
[H
+
] is critical in many chemical reactions.
A quick method of denoting [H
+
] is via pH.
By definition pH = log [H
+
], [H
+
] = 10
-pH
The pH scale, similar to the Richter scale,
describes a wide range of values
An earthquake of 6 is 10 as violent as a 5
Thus, the pH scale condenses possible
values of [H
+
] to a 14 point scale (fig. 2, p370)
Also, it is easier to say pH = 7 vs. [H
+
] = 1 x 10
7
Calculations with pH
Ans: 4.2
3.98 x 10
8
M
Try questions 2 and 6 (a-b) on page 375
Q: What is the pH if [H
+
]= 6.3 x 10
5
?
pH = log [H
+
]
(6.3, exp or EE, 5, +/-, log, +/-)
(-, log, 6.3, exp or EE, -, 5)
Q: What is the [H
+
] if pH = 7.4?
[H
+
] = 10
pH
mol/L
(10, x
y
, 7.4, +/-, =)
(10, ^, -, 7.4, =)
Pg. 375
2 a) pH = log [H
+
] = log [1x10
8
] = 8.0
b) pH = log [H
+
] = log [1x10
7
] = 7.0
c) pH = log [H
+
] = log [2.5x10
6
] = 5.60
d) pH = log [H
+
] = log [1.3x10
4
] = 3.89
6 a) [H
+
] = 10
pH
= 10
5.4
= 4 x 10
6
mol/L
b) [H
+
] = 10
pH
= 10
5.72
= 1.9 x 10
6
mol/L
Historical views on acids
The more recent Bronsted-Lowry concept is
that acids are H
+
(proton) donors and bases
are proton acceptors
Ionization
+ Cl H
H
H
O
+
H
H
H O
Cl
+
O (e.g. H
2
SO
4
) was originally thought to cause
acidic properties. Later, H was implicated, but
it was still not clear why CH
4
was neutral.
Arrhenius made the revolutionary suggestion
that some solutions contain ions & that acids
produce H
3
O
+
(hydronium) ions in solution.
The Bronsted-Lowry concept
In this idea, the ionization of an acid by water
is just one example of an acid-base reaction.
Acids and bases are identified based on
whether they donate or accept H
+
.
Conjugate acids and bases are found on the
products side of the equation. A conjugate
base is the same as the starting acid minus H
+
.
+ Cl H
H
H
O
+
H
H
H O
Cl
+
acid base conjugate acid conjugate base
conjugate acid-base pairs
Practice problems
Identify the acid, base, conjugate acid,
conjugate base, and conjugate acid-base pairs:
Reference: pg. 386 387
Try Q18 (p389), Q 8 & 11 (p392): do as above
acid base conjugate acid conjugate base
HC
2
H
3
O
2
(aq) + H
2
O(l) C
2
H
3
O
2
(aq) + H
3
O
+
(aq)
conjugate acid-base pairs
acid base conjugate acid conjugate base
OH
(aq) + HCO
3
(aq) CO
3
2
(aq) + H
2
O(l)
conjugate acid-base pairs
acid base conjugate acid conjugate base
HF(aq) + SO
3
2
(aq) F
(aq) + HSO
3
(aq)
conjugate acid-base pairs
acid base conjugate acid conjugate base
CO
3
2
(aq) + HC
2
H
3
O
2
(aq) C
2
H
3
O
2
(aq) + HCO
3
(aq)
conjugate acid-base pairs
acid base conjugate acid conjugate base
H
3
PO
4
(aq) + OCl
(aq) H
2
PO
4
(aq) + HOCl(aq)
conjugate acid-base pairs
Answers: question 18
(a)
(b)
(c)
acid base conjugate base conjugate acid
HCO
3
(aq) + S
2
(aq) HS
(aq) + CO
3
2
(aq)
conjugate acid-base pairs
base acid conjugate acid conjugate base
H
2
CO
3
(aq) + OH
(aq) HCO
3
(aq) + H
2
O(l)
conjugate acid-base pairs
acid base conjugate acid conjugate base
H
3
O
+
(aq) + HSO
3
(aq) H
2
O(l) + H
2
SO
3
(aq)
conjugate acid-base pairs
8a)
8b)
11a)
base acid conjugate base conjugate acid
OH
(aq) + HSO
3
(aq) H
2
O(l) + SO
3
2
(aq)
conjugate acid-base pairs
11b)
For more lessons, visit
www.chalkbored.com
Anda mungkin juga menyukai
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Excel 1Dokumen4 halamanExcel 1Branimir ZivanovicBelum ada peringkat
- Monitoring of The Human Resources Process - Automotive IndustryDokumen6 halamanMonitoring of The Human Resources Process - Automotive IndustryBranimir ZivanovicBelum ada peringkat
- Luti - MuriDokumen10 halamanLuti - MuriBranimir ZivanovicBelum ada peringkat
- Luti - MudaDokumen7 halamanLuti - MudaBranimir ZivanovicBelum ada peringkat
- Running Head: Human Resources Needs AssessmentDokumen26 halamanRunning Head: Human Resources Needs AssessmentBranimir ZivanovicBelum ada peringkat
- Internal Logistics Flow Simulation in Automotive IndustryDokumen22 halamanInternal Logistics Flow Simulation in Automotive IndustryBranimir ZivanovicBelum ada peringkat
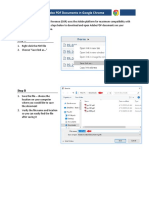
- Opening Adobe PDF Documents in Google Chrome: Step ADokumen2 halamanOpening Adobe PDF Documents in Google Chrome: Step ABranimir ZivanovicBelum ada peringkat
- How To Save Gmail As PDFDokumen3 halamanHow To Save Gmail As PDFAftab KhanBelum ada peringkat
- PDF TestDokumen1 halamanPDF TestBranimir ZivanovicBelum ada peringkat
- 1998 Testorone FIAT, SUPPLIERDokumen3 halaman1998 Testorone FIAT, SUPPLIERBranimir ZivanovicBelum ada peringkat
- Logistics 4.0 and Supply Chain 4.0 in The Automotive IndustryDokumen7 halamanLogistics 4.0 and Supply Chain 4.0 in The Automotive IndustryBranimir ZivanovicBelum ada peringkat
- 03 Cost Deplaoyment YamashinaDokumen16 halaman03 Cost Deplaoyment YamashinaBranimir ZivanovicBelum ada peringkat
- Reducing Urgent Shipping Costs by 68% for an Automotive Components ManufacturerDokumen17 halamanReducing Urgent Shipping Costs by 68% for an Automotive Components ManufacturerBranimir ZivanovicBelum ada peringkat
- Workplace Organisation As A Tool of RestructuringDokumen12 halamanWorkplace Organisation As A Tool of RestructuringBranimir ZivanovicBelum ada peringkat
- 03 Cost Deplaoyment YamashinaDokumen16 halaman03 Cost Deplaoyment YamashinaBranimir ZivanovicBelum ada peringkat
- World Class Manufacturing Model PDFDokumen8 halamanWorld Class Manufacturing Model PDFheldownBelum ada peringkat
- Ancient CivDokumen44 halamanAncient CivBranimir ZivanovicBelum ada peringkat
- World Class Manufacturing Model PDFDokumen8 halamanWorld Class Manufacturing Model PDFheldownBelum ada peringkat
- ArchemedisDokumen19 halamanArchemedisBranimir ZivanovicBelum ada peringkat
- Acids & Bases: by Robert McgeeDokumen12 halamanAcids & Bases: by Robert McgeeArumugam ManickamBelum ada peringkat
- Workplace Organisation As A Tool of RestructuringDokumen12 halamanWorkplace Organisation As A Tool of RestructuringBranimir ZivanovicBelum ada peringkat
- Atomic ModelsDokumen12 halamanAtomic ModelsBranimir ZivanovicBelum ada peringkat
- 14 Plant Kingdom Part TwoDokumen12 halaman14 Plant Kingdom Part TwoBranimir ZivanovicBelum ada peringkat
- 13 Plant Kingdom Part OneDokumen26 halaman13 Plant Kingdom Part OneBranimir ZivanovicBelum ada peringkat
- 011 LightDokumen24 halaman011 LightBranimir ZivanovicBelum ada peringkat
- Air ResistanceDokumen5 halamanAir ResistanceBranimir ZivanovicBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- A Stated-Preference Study of The Willingness-To-Pay To Reduce Traffic Risk in Urban vs. Rural RoadsDokumen13 halamanA Stated-Preference Study of The Willingness-To-Pay To Reduce Traffic Risk in Urban vs. Rural RoadsSantanu KumarBelum ada peringkat
- Sabp G 007Dokumen8 halamanSabp G 007Li PengBelum ada peringkat
- A e Regulator GuideDokumen1 halamanA e Regulator Guidenasser4858Belum ada peringkat
- Project PPTDokumen47 halamanProject PPTIshant KumawatBelum ada peringkat
- 24Dokumen3 halaman24sdssdBelum ada peringkat
- MBA (Travel & Tourism) 1st Year Sylabus 2020-21 - 28th SeptDokumen34 halamanMBA (Travel & Tourism) 1st Year Sylabus 2020-21 - 28th SeptHimanshuBelum ada peringkat
- CS5371 Theory of Computation: Lecture 1: Mathematics Review I (Basic Terminology)Dokumen23 halamanCS5371 Theory of Computation: Lecture 1: Mathematics Review I (Basic Terminology)Kamal WaliaBelum ada peringkat
- Solution of Problem Set 1 For Purity Hydrocarbon Data PDFDokumen4 halamanSolution of Problem Set 1 For Purity Hydrocarbon Data PDFDrumil TrivediBelum ada peringkat
- 2023-RegisterBook Version 202212Dokumen95 halaman2023-RegisterBook Version 202212Moin AltafBelum ada peringkat
- Brushless DC Motor Control Using PLCDokumen6 halamanBrushless DC Motor Control Using PLCvuluyen6688Belum ada peringkat
- Areas Related To CircleDokumen32 halamanAreas Related To CircleGiorno GiovannaBelum ada peringkat
- Liquefaction AnalysisDokumen16 halamanLiquefaction AnalysisKristi Engineering Services Pvt. Ltd.Belum ada peringkat
- DT022BTFT v10Dokumen12 halamanDT022BTFT v10Johny JongBelum ada peringkat
- Fundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0015Dokumen1 halamanFundamentals of Heat and Mass Transfer 7th Edition - Bergman, Lavine, Incropera, DeWitt (1) - p0015CladyBelum ada peringkat
- Operation Manual Zoomlion QY40Dokumen133 halamanOperation Manual Zoomlion QY40Hải Tiến100% (1)
- Akvola Technologies MicroGas S Technical Specifications - Web PDFDokumen2 halamanAkvola Technologies MicroGas S Technical Specifications - Web PDFHardik VavdiyaBelum ada peringkat
- Info Iec62271-1Dokumen28 halamanInfo Iec62271-1RichardLemusBelum ada peringkat
- Measuring AssignmentDokumen3 halamanMeasuring AssignmentArnab BhattacharyaBelum ada peringkat
- Mathematics T (954/1) Functions QuizDokumen1 halamanMathematics T (954/1) Functions QuizmasyatiBelum ada peringkat
- 1 Gauss SeidelDokumen20 halaman1 Gauss SeidelYanes Kristianus HediBelum ada peringkat
- QST HamClockDokumen3 halamanQST HamClockCPC PHCBelum ada peringkat
- Ultra-Low-Power Wireless Systems - Energy-Efficient Radios For The Internet of Things PDFDokumen11 halamanUltra-Low-Power Wireless Systems - Energy-Efficient Radios For The Internet of Things PDFnhatvpBelum ada peringkat
- Atlas Copco Compressed Air Manual: 8 EditionDokumen25 halamanAtlas Copco Compressed Air Manual: 8 EditionRajBelum ada peringkat
- CCR Load Calculator 2014-03-13Dokumen35 halamanCCR Load Calculator 2014-03-13Danielle FowlerBelum ada peringkat
- Operations Management 1St Edition Cachon Test Bank Full Chapter PDFDokumen36 halamanOperations Management 1St Edition Cachon Test Bank Full Chapter PDFwayne.martin885100% (11)
- Hibbeler D14 e CH 12 P 23Dokumen2 halamanHibbeler D14 e CH 12 P 23Mona fabrigarBelum ada peringkat
- Transformer X - R CalculationDokumen2 halamanTransformer X - R CalculationTharindu WimalasekaraBelum ada peringkat
- ACL Injuries in The Female Athlete: Causes, Impacts, and Conditioning Programs Frank R. Noyes Sue Barber-WestinDokumen658 halamanACL Injuries in The Female Athlete: Causes, Impacts, and Conditioning Programs Frank R. Noyes Sue Barber-WestinluizamgoBelum ada peringkat
- J Gen Physiol-1952-Hershey-39-56Dokumen18 halamanJ Gen Physiol-1952-Hershey-39-56api-277839406Belum ada peringkat
- Laminar Flow Reactor ProblemDokumen6 halamanLaminar Flow Reactor ProblemAileen Banua Añonuevo100% (1)