Bridion
Diunggah oleh
Eva Garcia MartinezHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Bridion
Diunggah oleh
Eva Garcia MartinezHak Cipta:
Format Tersedia
Bridion - Sugammadex
Manufacturer: Merck
FDA Approval Date: December 15, 2015
Bridion - Sugammadex
Objectives
At the end of this presentation
participants will be able to:
1. Appropriately recommend Bridion -
Sugammadex
2. Effectively educate patients on the
purpose, proper use and potential
adverse effects of Bridion Sugammadex
Bridion - Sugammadex
Clinical Application
Indications:
Used for adults undergoing surgery for the
reversal of neuromuscular blockade (NMB)
induced by rocuronium or vecuronium
Not indicated to reverse blockade of
nonsteroidal neuromuscular blocking
agents (eg, succinylcholine)
Not indicated for use in ICU setting
Bridion [package insert].
Bridion - Sugammadex
Clinical Application
Contraindications:
Hypersensitivity to sugammadex or any
component of the product
Precautions:
Use not recommended with severe renal
impairment or dialysis-dependent patients
Caution with mild-to-moderate renal
impairment (no dosage adjustment)
Bridion [package insert].
Bridion - Sugammadex
Clinical Application
Pregnancy:
No available human data
In animal studies, fetal birthweight was
reduced when 8x human dose was
administered
Lactation:
Unknown excretion in human breast milk
Present in animal milk
Bridion [package insert].
Bridion - Sugammadex
Drug Facts
Mechanism of Action
Cyclodextrin that forms a 1:1 complex
with rocuronium and vecuronium
This reduces the amount of free NMB
agent in plasma
In turn, the concentration gradient favors
movement of NMB agent away from the
neuromuscular junction
Bridion [package insert].
Bridion - Sugammadex
Drug Facts
Drug Interactions:
Hormonal contraceptives: must
recommend additional, non-hormonal
method of contraception for next 7 days
Toremifene: recovery can be delayed
Bridion [package insert].
Bridion - Sugammadex
Adverse Effects
Side effects are dose-dependent
Common (2-4 mg/kg) {16 mg/kg} [placebo]:
Nausea (23%) {26%} [23%]
Vomiting (11%) {15%} [10%]
Serious (2-4 mg/kg) {16 mg/kg} [placebo]:
Prolonged QT interval (1%) {6%} [1%]
Bradycardia (1%) {5%} [1%]
Hypotension (4%) {13%} [4%]
Anaphylaxis (0%) {1%} [0%]
Bridion [package insert].
Bridion - Sugammadex
Dosing Information
Dosing:
2-4 mg/kg IV bolus based on actual body
weight depending on blockade status
Immediate Reversal: 16 mg/kg IV bolus
based on actual body weight
This dose has only been studied for
rocuronium reversal
Bridion [package insert].
Bridion vs. neostigmine reversal agent for NMB
induced by rocuronium or vecuronium at
reappearance of T2 (Moderate Blockade)
Eur J Anaesthesiol 2001;18:99
Purpose: Comparing the efficacy of
sugammadex with neostigmine given with
glycopyrrolate or atropine in antagonizing block
produced by aminosteroidal NMBAs
Design:
Randomized, single-dose, active-controlled,
safety-assessor blinded trial of 98 patients
Sugammadex 2.0mg/kg or neostigmine 50
g/kg plus glycopyrrolate 10 g/kg were
administered intravenously in randomized
order
Blobner M, et al. Eur J Anaesthesiol. 2001;18:99.
Included: Adult patients scheduled for
surgery under general anesthesia in the
supine position
> 18 years old
ASA class 1 to 3
Primary outcome: Recovery time after
reversal agent administration
measured as train of four ratio (T4/T1)
of 0.9
Blobner M, et al. Eur J Anaesthesiol. 2001;18:99.
Serious adverse effects were
reported for 2 subjects in the
sugammadex group and 3 in the
neostigmine group
Adverse effects were not attributed
to the study drug
Blobner M, et al. Eur J Anaesthesiol. 2001;18:99.
Sugammadex achieved significantly
faster recovery of T4/T1 ratio to 0.9
compared with neostigmine after
neuromuscular block with rocuronium
Outliers and variability of recovery times
were significantly lower following
reversal with sugammadex
Blobner M, et al. Eur J Anaesthesiol. 2001;18:99.
Bridion - Sugammadex
Cost Comparison
Neuromuscular Blocking and Reversal Agents
AWP
Pricing
Sugammadex 200 mg/2 ml SDV
$1140
Sugammadex 500 mg/5 ml SDV
$2088
Neostigmine (Bloxiverz) 1 mg/ml 10 ml MDV
$1185
Pyridostigmine (Regonol) 5 mg/ml 2ml Vial
$384
Glycopyrrolate (Robinul) 0.2 mg/ml SDV
$390
AWP pricing as of 2/26/2016
Bridion - Sugammadex
Summary
Bridion , sugammadex, is first-in-class cyclodextrin
that directly reverses neuromuscular blockade
It is indicated for the reversal of neuromuscular
blockade for rocuronium and vecuronium for patients
undergoing surgical procedures only
Dosed: 2-4 mg/kg IV bolus based on total body weight
OR 16 mg/kg IV bolus reserved for emergent reversal
Offers faster reversal times compared to neostigmine
All women of child bearing age should be counseled
on using backup contraception for 7 days post-op
It is not cost prohibitive, but is more expensive than
neostigmine
Bridion - Sugammadex
References
1.
www.bridion.com
2.
Bridion package insert. Merck. Dec. 2015.
3.
Blobner M, Eriksson L, Scholz J, Hillebrand H, Pompei
L. Sugammadex (2.0 mg/kg) significantly faster
reverses shallow rocuronium-induced neuromuscular
blockade compared with neostigmine (50 g/kg). Eur J
Anaesthesiol 2001;18:99.
Bridion - Sugammadex
References
4.
Sugammadex: DrugDex. (2016). In Micromedex
(Columbia Basin College Library ed.) [Electronic
version].Greenwood Village, CO: Truven Health
Analytics. Retrieved March 21, 2016 from
http://www.micromedexsolutions.com/
5.
Neostigmine: DrugDex. (2016). In Micromedex
(Columbia Basin College Library ed.) [Electronic
version].Greenwood Village, CO: Truven Health
Analytics. Retrieved March 21, 2016 from
http://www.micromedexsolutions.com/
Anda mungkin juga menyukai
- Briviact Film-Coated Tablets Summary of Product CharacteristicsDokumen110 halamanBriviact Film-Coated Tablets Summary of Product CharacteristicsBendisDacicaBelum ada peringkat
- EMERGENCY DRUG STUDYDokumen3 halamanEMERGENCY DRUG STUDYGrace Santos MirandaBelum ada peringkat
- AcetaminophenDokumen2 halamanAcetaminophendrugcardref100% (1)
- Antimalarial DrugsDokumen7 halamanAntimalarial DrugsHilmanBelum ada peringkat
- AmloDokumen1 halamanAmloamy navajaBelum ada peringkat
- KetoconazoleDokumen2 halamanKetoconazoleMD. DELWAR HOSSAINBelum ada peringkat
- College of Nursing: Cebu Normal UniversityDokumen5 halamanCollege of Nursing: Cebu Normal UniversityChelsea WuBelum ada peringkat
- Indometacin MonographDokumen9 halamanIndometacin MonographQuiKe PvBelum ada peringkat
- Pharma CardsDokumen5 halamanPharma CardsazancheBelum ada peringkat
- Managing dextrose therapyDokumen2 halamanManaging dextrose therapySanket TelangBelum ada peringkat
- Drug study on chemotherapeutic alkylating agentsDokumen16 halamanDrug study on chemotherapeutic alkylating agentsPrincess CruzBelum ada peringkat
- Erythromycin Drug StudyDokumen2 halamanErythromycin Drug StudyJude LabajoBelum ada peringkat
- Flores Mary Jane Generic Drug ChartDokumen23 halamanFlores Mary Jane Generic Drug ChartKristine Dela CruzBelum ada peringkat
- PrednisoneDokumen3 halamanPrednisoneMaja DeraBelum ada peringkat
- MoxifloxacinDokumen23 halamanMoxifloxacinKaaghu Raghu Micha0% (1)
- Suxamethonium Chloride Injection BP Product Information SummaryDokumen8 halamanSuxamethonium Chloride Injection BP Product Information SummarynanaBelum ada peringkat
- Drug StudyDokumen1 halamanDrug Studykennethbote0% (1)
- Paracetamol Biogesic Analgesic AntipyreticDokumen8 halamanParacetamol Biogesic Analgesic AntipyreticGian Era100% (1)
- Case Pre Drug StudyDokumen5 halamanCase Pre Drug StudyJoule PeirreBelum ada peringkat
- Drug Study LCPDokumen3 halamanDrug Study LCPalleen_viaBelum ada peringkat
- DRUG-STUDY Butorphanol LRDR AngelicaRonquilloDokumen2 halamanDRUG-STUDY Butorphanol LRDR AngelicaRonquillokarl eiron delos santosBelum ada peringkat
- PethidineDokumen9 halamanPethidineAnonymous VZtcCECtBelum ada peringkat
- Drug Study: Davao Doctors College General Malvar ST., Davao City Nursing ProgramDokumen3 halamanDrug Study: Davao Doctors College General Malvar ST., Davao City Nursing ProgramJear RomeroBelum ada peringkat
- ImipramineDokumen1 halamanImipramineMuhammad ArsalanBelum ada peringkat
- Salbuterol Generic NameDokumen4 halamanSalbuterol Generic NamejunieBelum ada peringkat
- IFOSFAMIDEDokumen4 halamanIFOSFAMIDEErza GenatrikaBelum ada peringkat
- Amniotic Fluid EmbolismDokumen13 halamanAmniotic Fluid Embolismelfa riniBelum ada peringkat
- Meloxicam drug study overviewDokumen5 halamanMeloxicam drug study overviewABARAJBelum ada peringkat
- Tinidazole Is An AntiDokumen6 halamanTinidazole Is An AntiNoi Maya Anggrita SariBelum ada peringkat
- Drug Study On EPINEPHRINEDokumen6 halamanDrug Study On EPINEPHRINEshadow gonzalezBelum ada peringkat
- Drug StudyDokumen16 halamanDrug StudyMonica Luz FajardoBelum ada peringkat
- Epinephrine DrugStudy WWW Rnpedia ComDokumen4 halamanEpinephrine DrugStudy WWW Rnpedia ComIrish LigayaBelum ada peringkat
- The Format of This Leaflet Was Determined by The Ministry of Health and Its Content Was Checked and Approved by It On February 2016Dokumen10 halamanThe Format of This Leaflet Was Determined by The Ministry of Health and Its Content Was Checked and Approved by It On February 2016ddandan_2Belum ada peringkat
- Individualized Medication Form: Student Name Client Room # Dates Client Initials Med. DXDokumen3 halamanIndividualized Medication Form: Student Name Client Room # Dates Client Initials Med. DXjenn_RNBelum ada peringkat
- Clopidogrel Bisulfate - PlavixDokumen2 halamanClopidogrel Bisulfate - PlavixKristi WrayBelum ada peringkat
- FlagylDokumen3 halamanFlagylAdrianne BazoBelum ada peringkat
- Generic Name: Ceftriaxone Brand Name: (Kept Rix) IV, 1g, q12Dokumen5 halamanGeneric Name: Ceftriaxone Brand Name: (Kept Rix) IV, 1g, q12De Sesto Rhys CarloBelum ada peringkat
- Ketorolac PI PDFDokumen2 halamanKetorolac PI PDFintan kusumaningtyasBelum ada peringkat
- Isoprenaline Infusion 2016Dokumen3 halamanIsoprenaline Infusion 2016Glory Claudia KarundengBelum ada peringkat
- TrazodoneDokumen20 halamanTrazodoneAjay MehtaBelum ada peringkat
- © The Mechanism of Action of Anectine Involves What Appears To Be A "Persistent"Dokumen6 halaman© The Mechanism of Action of Anectine Involves What Appears To Be A "Persistent"kaye marie100% (1)
- DRUGSDokumen5 halamanDRUGSDanica EspejoBelum ada peringkat
- PethidineDokumen6 halamanPethidineAnonymous NQDRERPcjBelum ada peringkat
- Linezolid (Zyvox)Dokumen1 halamanLinezolid (Zyvox)EBelum ada peringkat
- NEW ZEALAND DATA SHEET PETHIDINE TABLETSDokumen13 halamanNEW ZEALAND DATA SHEET PETHIDINE TABLETSAnonymous NQDRERPcjBelum ada peringkat
- Xylocard PiDokumen11 halamanXylocard PiRamakant SharmaBelum ada peringkat
- Spinal Anes Drug StudyDokumen12 halamanSpinal Anes Drug StudyNicosia Mae FerrerBelum ada peringkat
- Generic Name:: Drug Name Indicatio N Mechanism of Action Side Effects Nursing ResponsibilitiesDokumen1 halamanGeneric Name:: Drug Name Indicatio N Mechanism of Action Side Effects Nursing Responsibilitiesgrace pitogoBelum ada peringkat
- Drug Study on CelecoxibDokumen11 halamanDrug Study on CelecoxibPrincess Brigitte R. PATE�ABelum ada peringkat
- Dexmedetomidine - Drug Information - UpToDateDokumen21 halamanDexmedetomidine - Drug Information - UpToDateRicardo Ortiz NovilloBelum ada peringkat
- HaemaccelinfDokumen9 halamanHaemaccelinfSisca YulistianaBelum ada peringkat
- CytotecDokumen1 halamanCytotecEmily ChengBelum ada peringkat
- Triamcinolone (Topical) - Drug InformationDokumen5 halamanTriamcinolone (Topical) - Drug InformationMauricio Sv0% (1)
- Esomeprazole (Nexium)Dokumen4 halamanEsomeprazole (Nexium)Roderick AgbuyaBelum ada peringkat
- DelavirdineDokumen2 halamanDelavirdineRosher Deliman JanoyanBelum ada peringkat
- TrihexyphenidylDokumen7 halamanTrihexyphenidylfachsyarBelum ada peringkat
- Hirschsprung’s Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsDari EverandHirschsprung’s Disease, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsBelum ada peringkat
- Sugammadex - PIDokumen15 halamanSugammadex - PIveliaberlianaBelum ada peringkat
- Annex I Summary of Product CharacteristicsDokumen28 halamanAnnex I Summary of Product CharacteristicsEmma SinBelum ada peringkat
- American Academy of PediatricsDokumen320 halamanAmerican Academy of PediatricsJuan Sebasatián Arjona100% (2)
- Protocolo y Necesidad de Kit para Toxicidad Sistémica Por Anestésicos Locales - Elsevier Enhanced ReaderDokumen1 halamanProtocolo y Necesidad de Kit para Toxicidad Sistémica Por Anestésicos Locales - Elsevier Enhanced ReaderEva Garcia MartinezBelum ada peringkat
- Anest Caudal UsgDokumen3 halamanAnest Caudal UsgEva Garcia MartinezBelum ada peringkat
- Via Aerea Dificil Prehospitalario PDFDokumen1 halamanVia Aerea Dificil Prehospitalario PDFEva Garcia MartinezBelum ada peringkat
- AMA Journal of Ethics: July 2018, Volume 20, Number 7: E655-663Dokumen9 halamanAMA Journal of Ethics: July 2018, Volume 20, Number 7: E655-663Eva Garcia MartinezBelum ada peringkat
- Asma y Sulfato de MagnesioDokumen1 halamanAsma y Sulfato de MagnesioEva Garcia MartinezBelum ada peringkat
- Guia de Anesetsia AsaDokumen43 halamanGuia de Anesetsia AsadentisdocBelum ada peringkat
- Guia de Anesetsia AsaDokumen43 halamanGuia de Anesetsia AsadentisdocBelum ada peringkat
- @anesthesia Books 2018 AnaesthesiaDokumen64 halaman@anesthesia Books 2018 AnaesthesiaEva Garcia MartinezBelum ada peringkat
- ASPEN Prácticas Seguras para La Nutrición EnteralDokumen89 halamanASPEN Prácticas Seguras para La Nutrición EnteralsofiaBelum ada peringkat
- ASPEN Prácticas Seguras para La Nutrición EnteralDokumen89 halamanASPEN Prácticas Seguras para La Nutrición EnteralsofiaBelum ada peringkat
- Advances in Antibiotic Therapy PDFDokumen13 halamanAdvances in Antibiotic Therapy PDFThaysa LimaBelum ada peringkat
- Advances in Antibiotic Therapy PDFDokumen13 halamanAdvances in Antibiotic Therapy PDFThaysa LimaBelum ada peringkat
- Advances in Antibiotic Therapy PDFDokumen13 halamanAdvances in Antibiotic Therapy PDFThaysa LimaBelum ada peringkat
- BOC ClaimDokumen3 halamanBOC ClaimWYBelum ada peringkat
- Zygoma Implant Procedure GuideDokumen63 halamanZygoma Implant Procedure GuideKristina Robles100% (2)
- NSAIDS ComparisonDokumen60 halamanNSAIDS ComparisonsillymajorBelum ada peringkat
- Protocol - Aminoglycoside DosingDokumen5 halamanProtocol - Aminoglycoside DosingThomasBelum ada peringkat
- 2021 ESMO Essentials For Clinicians Gastrointestinal Tract Tumours PDFDokumen133 halaman2021 ESMO Essentials For Clinicians Gastrointestinal Tract Tumours PDFCynthia LopezBelum ada peringkat
- Illness Anxiety DisorderDokumen2 halamanIllness Anxiety DisorderPrince AroraBelum ada peringkat
- Parasitology: Prepared By: Charriz A. AmoyanDokumen99 halamanParasitology: Prepared By: Charriz A. AmoyanRaphylyn EscalonaBelum ada peringkat
- Pain Pada SyringomyeliaDokumen6 halamanPain Pada Syringomyeliavico julendiBelum ada peringkat
- Endoscope Disinfection by SosDokumen12 halamanEndoscope Disinfection by SosSimran BajajBelum ada peringkat
- Hypokalemia and Hyperkalemia: Normal Value: 3.5-5 Meq/L Hypokalemia HyperkalemiaDokumen7 halamanHypokalemia and Hyperkalemia: Normal Value: 3.5-5 Meq/L Hypokalemia HyperkalemiaSyimah UmarBelum ada peringkat
- Common Procedures in Other Avian Species: Angela M. Lennox, DVM, DABVP-AvianDokumen17 halamanCommon Procedures in Other Avian Species: Angela M. Lennox, DVM, DABVP-AvianjudithBelum ada peringkat
- Anoxic Brain InjuryDokumen4 halamanAnoxic Brain InjuryRisky Ilona SaputraBelum ada peringkat
- Eye DisordersDokumen68 halamanEye DisordersYasmeen zulfiqarBelum ada peringkat
- Tooth Mobility IndicesDokumen7 halamanTooth Mobility Indiceshind bendahhaneBelum ada peringkat
- The Human Microbiome: by Unnati BhaleraoDokumen14 halamanThe Human Microbiome: by Unnati BhaleraoUnnati BhaleraoBelum ada peringkat
- Essential Stroke Nursing GuideDokumen5 halamanEssential Stroke Nursing GuideAshleigh Johnstone100% (1)
- Sindromul de Tunel CarpianDokumen46 halamanSindromul de Tunel CarpianMuresan Calin100% (2)
- No. 15 Casumpang-v-CortejoDokumen2 halamanNo. 15 Casumpang-v-CortejoGretchen Dominguez100% (1)
- UrticariaDokumen21 halamanUrticariaSumon GhoshBelum ada peringkat
- 103 Dosage Chart - tcm1310-72815 PDFDokumen1 halaman103 Dosage Chart - tcm1310-72815 PDFJaveria ShafiBelum ada peringkat
- Clinical Guideline For The Evaluation and Management of Chronic Insomnia in AdultsDokumen18 halamanClinical Guideline For The Evaluation and Management of Chronic Insomnia in AdultsDeTe Punya Map100% (1)
- Hip Fracture Risk Factors in Elderly WomanDokumen92 halamanHip Fracture Risk Factors in Elderly WomanAps CnjBelum ada peringkat
- Drug StudyDokumen8 halamanDrug StudyJuan de Vera100% (4)
- Q3-PPT-PE7-Week 1 (Concept of Folk Dance)Dokumen16 halamanQ3-PPT-PE7-Week 1 (Concept of Folk Dance)Patricia Anne SantosBelum ada peringkat
- DR Horst Filtzer AcidosisDokumen1 halamanDR Horst Filtzer AcidosisRon HipnerBelum ada peringkat
- Ureauv: 5 X 100/5 X 29 ML 12011025Dokumen1 halamanUreauv: 5 X 100/5 X 29 ML 12011025Ajish joBelum ada peringkat
- Diabetes - Lecture: Prof. Dr. Doina CatrinoiuDokumen131 halamanDiabetes - Lecture: Prof. Dr. Doina CatrinoiuPantea Constantin AlinBelum ada peringkat
- A Man'S Guide To Testosterone Replacement TherapyDokumen21 halamanA Man'S Guide To Testosterone Replacement TherapyEduardo NunesBelum ada peringkat
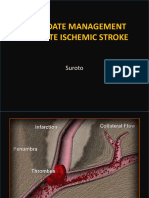
- An Update Management of Acute Ischemic Stroke: SurotoDokumen36 halamanAn Update Management of Acute Ischemic Stroke: SurotoShinta DianBelum ada peringkat
- Patient Assessment Phandhu Wicaksono 205070209111039 SAP 2020Dokumen3 halamanPatient Assessment Phandhu Wicaksono 205070209111039 SAP 2020Phandhu WicaksonoBelum ada peringkat