Konfigurasi Elektron Unsur Golongan A Oleh Urip Kalteng
Diunggah oleh
WarungOnlinekuJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Konfigurasi Elektron Unsur Golongan A Oleh Urip Kalteng
Diunggah oleh
WarungOnlinekuHak Cipta:
Format Tersedia
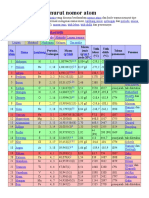
N
o
m
o
r
U
r
u
t
Nama Unsur
Lambang
Unsur
N
o
m
o
r
A
t
o
m
P
e
r
i
o
d
e
K(1) L(2) M(3) N(4) O(5) P(6) Q(7)
1 Hidrogen H 1 1 I A 1 1
2 Helium He 2 18 VIII A 1 2 L(2)
3 Litium Li 3 1 I A 2 2 1
4 Berilium Be 4 2 II A 2 2 2
5 Boron B 5 13 III A 2 2 3
6 Karbon C 6 14 IV A 2 2 4
7 Nitrogen N 7 15 V A 2 2 5
8 Oksigen O 8 16 VI A 2 2 6
9 Flor F 9 17 VII A 2 2 7
10 Neon Ne 10 18 VIII A 2 2 8 M(3)
11 Natrium Na 11 1 I A 3 2 8 1
12 Magnesium Mg 12 2 II A 3 2 8 2
13 Aluminum Al 13 13 III A 3 2 8 3
14 Silikon Si 14 14 IV A 3 2 8 4
15 Fosfor P 15 15 V A 3 2 8 5
16 Belerang S 16 16 VI A 3 2 8 6
17 Klor Cl 17 17 VII A 3 2 8 7
18 Argon Ar 18 18 VIII A 3 2 8 8 N(4)
19 Kalium K 19 1 I A 4 2 8 8 1
20 Kalsium Ca 20 2 II A 4 2 8 8 2
21 Gallium Ga 31 13 III A 4 2 8 18 3
22 Germanium Ge 32 14 IV A 4 2 8 18 4
23 Arsen As 33 15 V A 4 2 8 18 5
24 Selenium Se 34 16 VI A 4 2 8 18 6
25 Brom Br 35 17 VII A 4 2 8 18 7
26 Kripton Kr 36 18 VIII A 4 2 8 18 8 O(5)
27 Rubidium Rb 37 1 I A 5 2 8 18 8 1
28 Stronsium Sr 38 2 II A 5 2 8 18 8 2
29 Indium In 49 13 III A 5 2 8 18 18 3
30 Timah Sn 50 14 IV A 5 2 8 18 18 4
31 Antimon Sb 51 15 V A 5 2 8 18 18 5
32 Tellurium Te 52 16 VI A 5 2 8 18 18 6
33 Iod I 53 17 VII A 5 2 8 18 18 7
34 Xenon Xe 54 18 VIII A 5 2 8 18 18 8 P(6)
35 Cesium Cs 55 1 I A 6 2 8 18 18 8 1
36 Barium Ba 56 2 II A 6 2 8 18 18 8 2
37 Tallium Tl 81 13 III A 6 2 8 18 32 18 3
38 Timbal Pb 82 14 IV A 6 2 8 18 32 18 4
39 Bismuth Bi 83 15 V A 6 2 8 18 32 18 5
40 Polonium Po 84 16 VI A 6 2 8 18 32 18 6
41 Astatin At 85 17 VII A 6 2 8 18 32 18 7
42 Radon Rn 86 18 VIII A 6 2 8 18 32 18 8 Q(7)
43 Fransium Fr 87 1 I A 7 2 8 18 32 18 8 1
44 Radium Ra 88 2 II A 7 2 8 18 32 18 8 2
45 Ununtrium Uut 113 13 III A 7 2 8 18 32 32 18 3
46 Flerovium Fl 114 14 IV A 7 2 8 18 32 32 18 4
47 Ununpentium Uup 115 15 V A 7 2 8 18 32 32 18 5
48 Livermorium Lv 116 16 VI A 7 2 8 18 32 32 18 6
49 Ununseptium Uus 117 17 VII A 7 2 8 18 32 32 18 7
50 Ununoktium Uuo 118 18 VIII A 7 2 8 18 32 32 18 8
N
o
m
o
r
U
r
u
t
Nama Unsur
Lambang
Unsur
N
o
m
o
r
A
t
o
m
P
e
r
i
o
d
e
K(1) L(2) M(3) N(4) O(5) P(6) Q(7)
Disusun oleh Urip Kalteng (Blog Guru Kimia Borneo http://urip.wordpress.com)
G
o
l
o
n
g
a
n
KULIT ATOM KE
Disusun oleh Urip Kalteng (Blog Guru Kimia Borneo http://urip.wordpress.com)
KONFIGURASI ELEKTRON UNSUR GOLONGAN UTAMA (GOLONGAN A)
KULIT ATOM KE
G
o
l
o
n
g
a
n
Anda mungkin juga menyukai
- Daftar unsur kimia berdasarkan nomor atomDokumen8 halamanDaftar unsur kimia berdasarkan nomor atomArya BuanaBelum ada peringkat
- Tabel Periodik Unsur StandarDokumen11 halamanTabel Periodik Unsur Standarbiaseaje0% (1)
- Fiqa Dwiwardhina - 21251469 - Tugas 1 Plant DesignDokumen5 halamanFiqa Dwiwardhina - 21251469 - Tugas 1 Plant DesignFiqa DwiwardhinaBelum ada peringkat
- Tugas 1 Plant DesignDokumen9 halamanTugas 1 Plant DesignFiqa DwiwardhinaBelum ada peringkat
- Plant DesignDokumen5 halamanPlant DesignFiqa DwiwardhinaBelum ada peringkat
- Ikatan KimiaDokumen6 halamanIkatan KimiaAkhmad Rosul RaisBelum ada peringkat
- Soal Latihan Struktur Atom Dan Sistem Periodik - WEIN RADJADokumen3 halamanSoal Latihan Struktur Atom Dan Sistem Periodik - WEIN RADJAWein RadjaBelum ada peringkat
- Soal Latihan Struktur Atom Dan Sistem Periodik 2Dokumen3 halamanSoal Latihan Struktur Atom Dan Sistem Periodik 2Wein RadjaBelum ada peringkat
- Daftar Unsur Menurut NamaDokumen10 halamanDaftar Unsur Menurut Namawiwit suciatiBelum ada peringkat
- Daftar Unsur Menurut NamaDokumen10 halamanDaftar Unsur Menurut Namamuhammad gunturBelum ada peringkat
- Spu CDokumen3 halamanSpu CAllan GunawanBelum ada peringkat
- Daftar_unsur_kimiaDokumen8 halamanDaftar_unsur_kimiaIndahBelum ada peringkat
- Daftar Unsur Menurut Nomor AtomDokumen56 halamanDaftar Unsur Menurut Nomor AtomsupriyadiBelum ada peringkat
- Konfigurasi Elektron Dan Susunan BerkalaDokumen7 halamanKonfigurasi Elektron Dan Susunan BerkalaAF SpectreBelum ada peringkat
- DAFTAR UNSURDokumen8 halamanDAFTAR UNSURYoga WinataBelum ada peringkat
- Review Kimia Sistem Periodik UnsurDokumen5 halamanReview Kimia Sistem Periodik UnsurLaras PutriBelum ada peringkat
- Sistem Periodik UnsurDokumen30 halamanSistem Periodik UnsurAriesta CrisandanaBelum ada peringkat
- Tabel KimiaDokumen2 halamanTabel KimiaHarisBelum ada peringkat
- Daftar Unsur Menurut Nomor AtomDokumen3 halamanDaftar Unsur Menurut Nomor AtomPutri AurelBelum ada peringkat
- SEMUA ZATDokumen35 halamanSEMUA ZATErwin SaputraBelum ada peringkat
- Sistem Periodik Unsur PDFDokumen48 halamanSistem Periodik Unsur PDFReski Mar'afBelum ada peringkat
- Daftar Unsur Menurut Nomor AtomDokumen10 halamanDaftar Unsur Menurut Nomor AtomAgus ParwataBelum ada peringkat
- Michael Yohannes Situngkir - Kimia DasarDokumen1 halamanMichael Yohannes Situngkir - Kimia Dasarmichaelightning23Belum ada peringkat
- BAB I - SalinDokumen3 halamanBAB I - SalinMas Habib RiyadiBelum ada peringkat
- Kimia Periode KetigaDokumen23 halamanKimia Periode Ketigasiti khansa nur aisyahBelum ada peringkat
- Konfigurasi ElektronDokumen30 halamanKonfigurasi ElektronbryansBelum ada peringkat
- Sistem PeriodikDokumen36 halamanSistem PeriodikRoihan 22Belum ada peringkat
- TabelPeriodikDokumen5 halamanTabelPeriodikadisti160285Belum ada peringkat
- ALKALI TANAHDokumen20 halamanALKALI TANAHnadiaBelum ada peringkat
- Kelimpahan UnsurDokumen13 halamanKelimpahan Unsurjaenul_swaggyBelum ada peringkat
- Nama Bahan Kimia Sma 4Dokumen3 halamanNama Bahan Kimia Sma 4rini kristiyantiBelum ada peringkat
- Elektron ValensiDokumen2 halamanElektron ValensiFinasyifa AfidahBelum ada peringkat
- KELIMPAHAN UNSURDokumen15 halamanKELIMPAHAN UNSURLia IndrawatiBelum ada peringkat
- Konfigurasi ElektronDokumen1 halamanKonfigurasi ElektronLidia Putri RamadhaniBelum ada peringkat
- Kumpulan Teori AnorganikDokumen44 halamanKumpulan Teori Anorganiknied1707Belum ada peringkat
- Daftar Unsur Menurut Nomor AtomDokumen6 halamanDaftar Unsur Menurut Nomor AtomMila HailiBelum ada peringkat
- Latihan Soal US PAKET ADokumen6 halamanLatihan Soal US PAKET AMedhira MandaBelum ada peringkat
- Daftar Unsur Kimia Menurut Nomor AtomDokumen5 halamanDaftar Unsur Kimia Menurut Nomor Atomᮓᮔᮤᮚᮜ᮪ᮃᮂᮙᮓ᮪ᮛᮤᮐᮜ᮪ᮓᮂᮤBelum ada peringkat
- 11 12Dokumen27 halaman11 12Dewi Miftahul JannahBelum ada peringkat
- Golongan Kalkogen OksigenDokumen22 halamanGolongan Kalkogen OksigenAna NanaBelum ada peringkat
- Unsur Periode 4Dokumen18 halamanUnsur Periode 4Mokhammad Brian YusufBelum ada peringkat
- LIGAN PEMBENTUK KELATDokumen40 halamanLIGAN PEMBENTUK KELATFatma MaharaniBelum ada peringkat
- Takwim 2010Dokumen1 halamanTakwim 2010rosdiembongBelum ada peringkat
- Tabel Unsur Kimia Dan LambangnyaDokumen10 halamanTabel Unsur Kimia Dan Lambangnyaumiyatul fahrini100% (1)
- Latihan Soal Olimpiade Ipa SMP 6Dokumen12 halamanLatihan Soal Olimpiade Ipa SMP 6Gina noordianaBelum ada peringkat
- BAB II-benar-1Dokumen32 halamanBAB II-benar-1aurora borealisBelum ada peringkat
- Tabel Unsur Kimia dan LambangnyaDokumen2 halamanTabel Unsur Kimia dan LambangnyatikaBelum ada peringkat
- BEPDokumen1 halamanBEPSMAN 1 MESUJI RAYABelum ada peringkat
- Tugas Administrasi Pergudangan Susunan Zat KimiaDokumen5 halamanTugas Administrasi Pergudangan Susunan Zat KimiaNisa SalsabilaBelum ada peringkat
- Lampiran 10 Tabel MasterDokumen2 halamanLampiran 10 Tabel MasterAgustiniBelum ada peringkat
- Daftar Bahan KimiaDokumen5 halamanDaftar Bahan KimiaennnezzzBelum ada peringkat
- Unsur Dan LambangnyaDokumen6 halamanUnsur Dan LambangnyaBolo PendemBelum ada peringkat
- Unsur, Senyawa, Dan CampuranDokumen7 halamanUnsur, Senyawa, Dan CampuranDwi DesdyBelum ada peringkat
- Table Unsur UnsurDokumen7 halamanTable Unsur UnsurNurul FarihahBelum ada peringkat
- Berat Atom dan Unsur KimiaDokumen3 halamanBerat Atom dan Unsur KimiaAhmad DayrobbiBelum ada peringkat
- Proses KLM (Final)Dokumen129 halamanProses KLM (Final)aisahBelum ada peringkat
- Tabel Periodik ElektronDokumen103 halamanTabel Periodik ElektronEkanisaKurniawatiBelum ada peringkat
- UNSURTRANSISIDokumen136 halamanUNSURTRANSISILena Sie ToettoetBelum ada peringkat
- BukuSMA Jilid1 2002 KrtsA4Dokumen208 halamanBukuSMA Jilid1 2002 KrtsA4MuchlisBelum ada peringkat